Comparative proteomics of exosomes secreted by tumoral Jurkat T cells and normal human T cell blasts unravels a potential tumorigenic role for valosin-containing protein
- PMID: 27086912
- PMCID: PMC5045396
- DOI: 10.18632/oncotarget.8678
Comparative proteomics of exosomes secreted by tumoral Jurkat T cells and normal human T cell blasts unravels a potential tumorigenic role for valosin-containing protein
Abstract
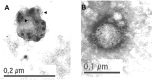
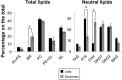
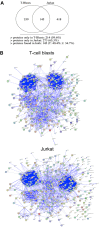
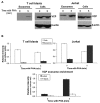
We have previously characterized that FasL and Apo2L/TRAIL are stored in their bioactive form inside human T cell blasts in intraluminal vesicles present in multivesicular bodies. These vesicles are rapidly released to the supernatant in the form of exosomes upon re-activation of T cells. In this study we have compared for the first time proteomics of exosomes produced by normal human T cell blasts with those produced by tumoral Jurkat cells, with the objective of identify proteins associated with tumoral exosomes that could have a previously unrecognized role in malignancy. We have identified 359 and 418 proteins in exosomes from T cell blasts and Jurkat cells, respectively. Interestingly, only 145 (around a 40%) are common. The major proteins in both cases are actin and tubulin isoforms and the common interaction nodes correspond to these cytoskeleton and related proteins, as well as to ribosomal and mRNA granule proteins. We detected 14 membrane proteins that were especially enriched in exosomes from Jurkat cells as compared with T cell blasts. The most abundant of these proteins was valosin-containing protein (VCP), a membrane ATPase involved in ER homeostasis and ubiquitination. In this work, we also show that leukemic cells are more sensitive to cell death induced by the VCP inhibitor DBeQ than normal T cells. Furthermore, VCP inhibition prevents functional exosome secretion only in Jurkat cells, but not in T cell blasts. These results suggest VCP targeting as a new selective pathway to exploit in cancer treatment to prevent tumoral exosome secretion.
Keywords: T cells; apoptosis; exosomes; leukemia; proteomics.
Conflict of interest statement
No conflict of interest to declare.
Figures



Similar articles
-
Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus.J Immunol. 2013 Dec 1;191(11):5515-23. doi: 10.4049/jimmunol.1301885. Epub 2013 Nov 1. J Immunol. 2013. PMID: 24184557
-
The role of cbl family of ubiquitin ligases in gastric cancer exosome-induced apoptosis of Jurkat T cells.Acta Oncol. 2009;48(8):1173-80. doi: 10.3109/02841860903032817. Acta Oncol. 2009. PMID: 19863226
-
A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes.Biochimie. 2007 Feb;89(2):213-21. doi: 10.1016/j.biochi.2006.07.018. Epub 2006 Aug 22. Biochimie. 2007. PMID: 16989932
-
Prions and exosomes: from PrPc trafficking to PrPsc propagation.Blood Cells Mol Dis. 2005 Sep-Oct;35(2):143-8. doi: 10.1016/j.bcmd.2005.06.013. Blood Cells Mol Dis. 2005. PMID: 16099696 Review.
-
Proteomic profiling of exosomes: current perspectives.Proteomics. 2008 Oct;8(19):4083-99. doi: 10.1002/pmic.200800109. Proteomics. 2008. PMID: 18780348 Review.
Cited by
-
Extracellular vesicles biogenesis, isolation, manipulation and genetic engineering for potential in vitro and in vivo therapeutics: An overview.Front Bioeng Biotechnol. 2022 Nov 4;10:1019821. doi: 10.3389/fbioe.2022.1019821. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36406206 Free PMC article. Review.
-
Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy.Cancers (Basel). 2019 Mar 29;11(4):444. doi: 10.3390/cancers11040444. Cancers (Basel). 2019. PMID: 30934872 Free PMC article. Review.
-
The roles of small extracellular vesicles in cancer and immune regulation and translational potential in cancer therapy.J Exp Clin Cancer Res. 2022 Sep 27;41(1):286. doi: 10.1186/s13046-022-02492-1. J Exp Clin Cancer Res. 2022. PMID: 36167539 Free PMC article. Review.
-
Extracellular Vesicles and Their Role in the Spatial and Temporal Expansion of Tumor-Immune Interactions.Int J Mol Sci. 2021 Mar 25;22(7):3374. doi: 10.3390/ijms22073374. Int J Mol Sci. 2021. PMID: 33806053 Free PMC article. Review.
-
SILAC-Based Quantification of TGFBR2-Regulated Protein Expression in Extracellular Vesicles of Microsatellite Unstable Colorectal Cancers.Int J Mol Sci. 2019 Aug 26;20(17):4162. doi: 10.3390/ijms20174162. Int J Mol Sci. 2019. PMID: 31454892 Free PMC article.
References
-
- Schrier SL, Godin D, Gould RG, Swyryd B, Junga I, Seeger M. Characterization of microvesicles produced by shearing of human erythrocyte membranes. Biochim Biophys Acta. 1971;233:26–36. - PubMed
-
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. - PubMed
-
- Johnstone RM, Ahn J. A common mechanism may be involved in the selective loss of plasma membrane functions during reticulocyte maturation. Biochim Biophys Acta. 1990;49:S70–75. - PubMed
-
- Dalton AJ. Microvesicles and vesicles of multivesicular bodies verus “virus-like” particles. J Natl Cancer Inst. 1975;54:1137–1148. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

