Arterial thrombosis in the context of HCV-associated vascular disease can be prevented by protein C
- PMID: 27086952
- PMCID: PMC5719134
- DOI: 10.1038/cmi.2016.10
Arterial thrombosis in the context of HCV-associated vascular disease can be prevented by protein C
Abstract
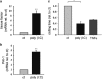
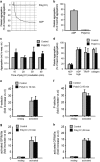
Hepatitis C virus (HCV) infection is a major problem worldwide. HCV is not limited to liver disease but is frequently complicated by immune-mediated extrahepatic manifestations such as glomerulonephritis or vasculitis. A fatal complication of HCV-associated vascular disease is thrombosis. Polyriboinosinic:polyribocytidylic acid (poly (I:C)), a synthetic analog of viral RNA, induces a Toll-like receptor 3 (TLR3)-dependent arteriolar thrombosis without significant thrombus formation in venules in vivo. These procoagulant effects are caused by increased endothelial synthesis of tissue factor and PAI-1 without platelet activation. In addition to human umbilical endothelial cells (HUVEC), human mesangial cells (HMC) produce procoagulatory factors, cytokines and adhesion molecules after stimulation with poly (I:C) or HCV-containing cryoprecipitates from a patient with a HCV infection as well. Activated protein C (APC) is able to prevent the induction of procoagulatory factors in HUVEC and HMC in vitro and blocks the effects of poly (I:C) and HCV-RNA on the expression of cytokines and adhesion molecules in HMC but not in HUVEC. In vivo, protein C inhibits poly (I:C)-induced arteriolar thrombosis. Thus, endothelial cells are de facto able to actively participate in immune-mediated vascular thrombosis caused by viral infections. Finally, we provide evidence for the ability of protein C to inhibit TLR3-mediated arteriolar thrombosis caused by HCV infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
TLR3-dependent immune regulatory functions of human mesangial cells.Cell Mol Immunol. 2012 Jul;9(4):334-40. doi: 10.1038/cmi.2012.3. Epub 2012 Apr 2. Cell Mol Immunol. 2012. PMID: 22466005 Free PMC article.
-
Novel role of toll-like receptor 3 in hepatitis C-associated glomerulonephritis.Am J Pathol. 2006 Feb;168(2):370-85. doi: 10.2353/ajpath.2006.050491. Am J Pathol. 2006. PMID: 16436653 Free PMC article.
-
Hepatitis C virus induced endothelial inflammatory response depends on the functional expression of TNFα receptor subtype 2.PLoS One. 2014 Nov 24;9(11):e113351. doi: 10.1371/journal.pone.0113351. eCollection 2014. PLoS One. 2014. PMID: 25419735 Free PMC article.
-
Hepatitis C and renal disease: epidemiology, diagnosis, pathogenesis and therapy.Contrib Nephrol. 2012;176:10-23. doi: 10.1159/000333772. Epub 2012 Jan 30. Contrib Nephrol. 2012. PMID: 22310777 Review.
-
Innate immune responses in hepatitis C virus infection.Semin Immunopathol. 2013 Jan;35(1):53-72. doi: 10.1007/s00281-012-0332-x. Epub 2012 Aug 7. Semin Immunopathol. 2013. PMID: 22868377 Free PMC article. Review.
Cited by
-
Perivascular Mesenchymal Stem/Stromal Cells, an Immune Privileged Niche for Viruses?Int J Mol Sci. 2022 Jul 21;23(14):8038. doi: 10.3390/ijms23148038. Int J Mol Sci. 2022. PMID: 35887383 Free PMC article. Review.
-
Potential Immunoregulatory Mechanism of Plant Saponins: A Review.Molecules. 2023 Dec 23;29(1):113. doi: 10.3390/molecules29010113. Molecules. 2023. PMID: 38202696 Free PMC article. Review.
-
Double-stranded DNA induces a prothrombotic phenotype in the vascular endothelium.Sci Rep. 2017 Apr 25;7(1):1112. doi: 10.1038/s41598-017-01148-x. Sci Rep. 2017. PMID: 28442771 Free PMC article.
-
Role of Platelets in Detection and Regulation of Infection.Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):70-78. doi: 10.1161/ATVBAHA.120.314645. Epub 2020 Oct 29. Arterioscler Thromb Vasc Biol. 2021. PMID: 33115274 Free PMC article. Review.
-
Inhibitors of Polyphosphate and Neutrophil Extracellular Traps.Semin Thromb Hemost. 2024 Oct;50(7):970-977. doi: 10.1055/s-0043-1768936. Epub 2023 May 16. Semin Thromb Hemost. 2024. PMID: 37192652 Review.
References
-
- Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet 2003; 362: 2095–2100. - PubMed
-
- Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol 2009; 4: 207–220. - PubMed
-
- Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med 1992; 327: 1490–1495. - PubMed
-
- Ferri C, Greco F, Longombardo G, Palla P, Moretti A, Marzo E et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum 1991; 34: 1606–1610. - PubMed
-
- Cacoub P, Fabiani FL, Musset L, Perrin M, Frangeul L, Leger JM et al. Mixed cryoglobulinemia and hepatitis C virus. Am J Med 1994; 96: 124–132. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

