Oral fumaric acid esters for psoriasis: abridged Cochrane systematic review including GRADE assessments
- PMID: 27087044
- PMCID: PMC5095877
- DOI: 10.1111/bjd.14676
Oral fumaric acid esters for psoriasis: abridged Cochrane systematic review including GRADE assessments
Abstract
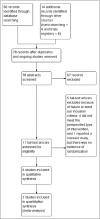
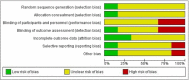
Fumaric acid esters (FAEs) are licensed for the treatment of moderate-to-severe psoriasis in Germany but are also used off-label in many other countries. We conducted this systematic review to synthesize the highest-quality evidence for the benefits and risks of FAEs for psoriasis. Our primary outcomes were change in Psoriasis Area and Severity Index score and dropout rates due to adverse effects. Randomized controlled trials (RCTs) of FAEs or dimethylfumarate were included, with no restriction on age or psoriasis subtype. We searched the Cochrane Skin Group Specialised Register, CENTRAL in the Cochrane Library, Medline, Embase, LILACS and five trials registers, and hand searched six conference proceedings. Six RCTs with a total of 544 participants were included, four of which were published only as abstracts or brief reports, limiting study reporting. Five RCTs compared FAEs with placebo, and all demonstrated benefit in favour of FAEs. However, meta-analysis was possible only for PASI 50 response after 12-16 weeks, which was achieved by 64% of participants on FAEs compared with 14% on placebo: risk ratio (RR) 4·55, 95% confidence interval (CI) 2·80-7·40; two studies; 247 participants; low-quality evidence). There was no difference in dropout rates due to adverse effects (RR 5·36, 95% CI 0·28-102·12; one study; 27 participants; very low-quality evidence and wide CI). More participants experienced nuisance adverse effects with FAEs (76%) than with placebo (16%) (RR 4·72, 95% CI 2·45-9·08; one study; 99 participants; moderate-quality evidence), mainly abdominal pain, diarrhoea and flushing. One head-to-head study of very low-quality evidence comparing FAEs with methotrexate reported comparable efficacy and dropout rates, although FAEs caused more flushing. The evidence in this review was limited and must be interpreted with caution; studies with better design and outcome reporting are needed.
© 2016 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures



Comment in
-
Fumarates for treatment of psoriasis.Br J Dermatol. 2016 Nov;175(5):857. doi: 10.1111/bjd.14944. Br J Dermatol. 2016. PMID: 27790679 No abstract available.
References
-
- Lebwohl M. Psoriasis. Lancet 2003; 361:1197–204. - PubMed
-
- Schweckendiek W. Treatment of psoriasis vulgaris. Med Monatsschr 1959; 13:103–4. - PubMed
-
- Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br J Dermatol 1999; 141:424–9. - PubMed
-
- Mrowietz U, Asadullah K. Dimethylfumarate for psoriasis: more than a dietary curiosity. Trends Mol Med 2005; 11:43–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

