Considerations in the Development of Reversibly Binding PET Radioligands for Brain Imaging
- PMID: 27087244
- PMCID: PMC5579844
- DOI: 10.2174/0929867323666160418114826
Considerations in the Development of Reversibly Binding PET Radioligands for Brain Imaging
Abstract
The development of reversibly binding radioligands for imaging brain proteins in vivo, such as enzymes, neurotransmitter transporters, receptors and ion channels, with positron emission tomography (PET) is keenly sought for biomedical studies of neuropsychiatric disorders and for drug discovery and development, but is recognized as being highly challenging at the medicinal chemistry level. This article aims to compile and discuss the main considerations to be taken into account by chemists embarking on programs of radioligand development for PET imaging of brain protein targets.
Conflict of interest statement
The author confirms that this article has no conflict of interest.
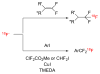
Figures











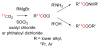

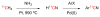
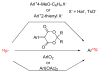

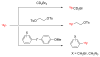
References
-
- Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nature Rev. 2007;5:821–834. - PubMed
-
- Heiss WD, Herholz K. Brain receptor imaging. J Nucl Med. 2006;47:302–312. - PubMed
-
- Politis M, Piccini P. Positron emission tomography imaging in neurological disorders. J Neurol. 2012;259:1769–1780. - PubMed
-
- Savitz JB, Drevets WC. Neuroreceptor imaging in depression. Neurobiol Dis. 2013;52:49–65. - PubMed
-
- Hargreaves RJ. The role of molecular imaging in drug discovery and development. Clin Pharmacol Ther. 2008;83:349–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

