Daily iron supplementation for improving anaemia, iron status and health in menstruating women
- PMID: 27087396
- PMCID: PMC10182438
- DOI: 10.1002/14651858.CD009747.pub2
Daily iron supplementation for improving anaemia, iron status and health in menstruating women
Abstract
Background: Iron-deficiency anaemia is highly prevalent among non-pregnant women of reproductive age (menstruating women) worldwide, although the prevalence is highest in lower-income settings. Iron-deficiency anaemia has been associated with a range of adverse health outcomes, which restitution of iron stores using iron supplementation has been considered likely to resolve. Although there have been many trials reporting effects of iron in non-pregnant women, these trials have never been synthesised in a systematic review.
Objectives: To establish the evidence for effects of daily supplementation with iron on anaemia and iron status, as well as on physical, psychological and neurocognitive health, in menstruating women.
Search methods: In November 2015 we searched CENTRAL, Ovid MEDLINE, EMBASE, and nine other databases, as well as four digital thesis repositories. In addition, we searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and reference lists of relevant reviews.
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs comparing daily oral iron supplementation with or without a cointervention (folic acid or vitamin C), for at least five days per week at any dose, to control or placebo using either individual- or cluster-randomisation. Inclusion criteria were menstruating women (or women aged 12 to 50 years) reporting on predefined primary (anaemia, haemoglobin concentration, iron deficiency, iron-deficiency anaemia, all-cause mortality, adverse effects, and cognitive function) or secondary (iron status measured by iron indices, physical exercise performance, psychological health, adherence, anthropometric measures, serum/plasma zinc levels, vitamin A status, and red cell folate) outcomes.
Data collection and analysis: We used the standard methodological procedures of Cochrane.
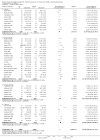
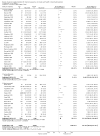
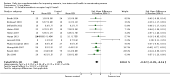
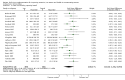
Main results: The search strategy identified 31,767 records; after screening, 90 full-text reports were assessed for eligibility. We included 67 trials (from 76 reports), recruiting 8506 women; the number of women included in analyses varied greatly between outcomes, with endpoint haemoglobin concentration being the outcome with the largest number of participants analysed (6861 women). Only 10 studies were considered at low overall risk of bias, with most studies presenting insufficient details about trial quality.Women receiving iron were significantly less likely to be anaemic at the end of intervention compared to women receiving control (risk ratio (RR) 0.39 (95% confidence interval (CI) 0.25 to 0.60, 10 studies, 3273 women, moderate quality evidence). Women receiving iron had a higher haemoglobin concentration at the end of intervention compared to women receiving control (mean difference (MD) 5.30, 95% CI 4.14 to 6.45, 51 studies, 6861 women, high quality evidence). Women receiving iron had a reduced risk of iron deficiency compared to women receiving control (RR 0.62, 95% CI 0.50 to 0.76, 7 studies, 1088 women, moderate quality evidence). Only one study (55 women) specifically reported iron-deficiency anaemia and no studies reported mortality. Seven trials recruiting 901 women reported on 'any side effect' and did not identify an overall increased prevalence of side effects from iron supplements (RR 2.14, 95% CI 0.94 to 4.86, low quality evidence). Five studies recruiting 521 women identified an increased prevalence of gastrointestinal side effects in women taking iron (RR 1.99, 95% CI 1.26 to 3.12, low quality evidence). Six studies recruiting 604 women identified an increased prevalence of loose stools/diarrhoea (RR 2.13, 95% CI 1.10, 4.11, high quality evidence); eight studies recruiting 1036 women identified an increased prevalence of hard stools/constipation (RR 2.07, 95% CI 1.35 to 3.17, high quality evidence). Seven studies recruiting 1190 women identified evidence of an increased prevalence of abdominal pain among women randomised to iron (RR 1.55, 95% CI 0.99 to 2.41, low quality evidence). Eight studies recruiting 1214 women did not find any evidence of an increased prevalence of nausea among women randomised to iron (RR 1.19, 95% CI 0.78 to 1.82). Evidence that iron supplementation improves cognitive performance in women is uncertain, as studies could not be meta-analysed and individual studies reported conflicting results. Iron supplementation improved maximal and submaximal exercise performance, and appears to reduce symptomatic fatigue. Although adherence could not be formally meta-analysed due to differences in reporting, there was no evident difference in adherence between women randomised to iron and control.
Authors' conclusions: Daily iron supplementation effectively reduces the prevalence of anaemia and iron deficiency, raises haemoglobin and iron stores, improves exercise performance and reduces symptomatic fatigue. These benefits come at the expense of increased gastrointestinal symptomatic side effects.
Conflict of interest statement
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclaimer: Luz Maria De‐Regil is a full‐time staff member of the Micronutrient Initiative. Jo Speedy, Claire Styles and Sant‐Rayn Pasricha are staff of the Australian Red Cross Blood Service. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the official position, decisions, policy or views of these organisations.
Figures
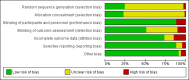
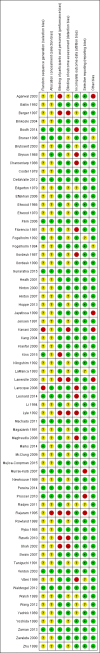












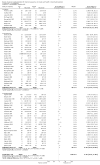

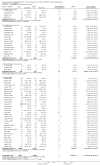
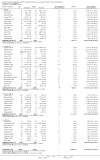
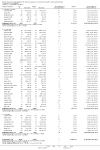





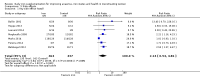






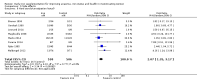

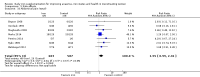
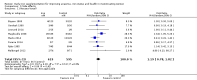


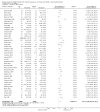
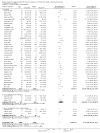

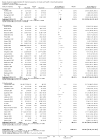
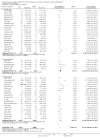




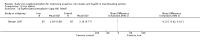
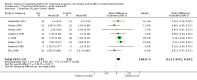

















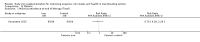
Update of
References
References to studies included in this review
Agarwal 2003 {published data only}
-
- Agarwal KN, Gomber S, Bisht H, Som M. Anemia prophylaxis in adolescent school girls by weekly or daily iron‐folate supplementation. Indian Pediatrics 2003;40(4):296‐301. [PUBMED: 12736400] - PubMed
Ballin 1992 {published data only}
-
- Ballin A, Berar M, Rubinstein U, Kleter Y, Hershkovitz A, Meytes D. Iron state in female adolescents. American Journal of Diseases of Children 1992;146(7):803‐5. - PubMed
Berger 1997 {published data only}
-
- Berger J, Aguayo VM, San Miguel JL, Lujan C, Tellez W, Traissac P. Definition and prevalence of anemia in Bolivian women of childbearing age living at high altitudes: the effect of iron‐folate supplementation. Nutrition Reviews 1997;55(6):247‐56. [DOI: 10.1111/j.1753-4887.1997.tb01612.x] - DOI - PubMed
Binkoski 2004 {published data only}
-
- Binkoski AE, Kris‐Etherton PM, Beard JL. Iron supplementation does not affect the susceptibility of LDL to oxidative modification in women with low iron status. Journal of Nutrition 2004; Vol. 134, issue 1:99‐103. [PUBMED: 14704300] - PubMed
Booth 2014 {published data only}
-
- Booth CK, Carins JE, Robertson IK. Randomised double‐blind, placebo‐controlled trial of iron supplementation attenuates fatigue and declining iron stores for female officers‐in‐training. Journal of Military & Veterans' Health 2014;22(3):13‐24.
Bruner 1996 {published data only}
-
- Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. Randomised study of cognitive effects of iron supplementation in non‐anaemic iron‐deficient adolescent girls. Lancet 1996;348(9033):992‐6. [PUBMED: 8855856] - PubMed
Brutsaert 2003 {published data only}
-
- Brutsaert TD, Hernandez‐Cordero S, Rivera J, Viola T, Hughes G, Haas JD. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron‐depleted, nonanemic women. American Journal of Clinical Nutrition 2003;77(2):441‐8. - PubMed
Bryson 1968 {published data only}
-
- Bryson DD. Iron and anaemia in adolescent girls: a double‐blind trial. Practitioner 1968;200(199):694‐7. - PubMed
Charoenlarp 1988 {published data only}
-
- Charoenlarp P, Dhanamitta S, Kaewvichit R, Silprasert A, Suwanaradd C, Na‐Nakorn S, et al. A WHO collaborative study on iron supplementation in Burma and in Thailand. American Journal of Clinical Nutrition 1988;47(2):280‐97. - PubMed
Cooter 1978 {published data only}
-
- Cooter RG, Mowbray KW. Effects of iron supplementation and activity on serum iron depletion and hemoglobin levels in female athletes. Research Quarterly 1978;49(2):114‐8. - PubMed
DellaValle 2012 {unpublished data only}
-
- DellaValle DM [pers comm]. The effects of iron depletion without anemia on training and performance in female collegiate rowers [personal communication]. Email to: SR Pasrischa 10 May 2012.
-
- DellaValle DM, Haas JD. Iron supplementation improves energetic efficiency in iron‐depleted female rowers. Medicine and Science in Sports and Exercise 2014;46(6):1204‐15. [PUBMED: 24195864 ] - PubMed
Edgerton 1979 {published data only}
Eftekhari 2006 {published data only}
-
- Eftekhari MH, Simondon KB, Jalali M, Keshavarz SA, Elguero E, Eshraghian MR, et al. Effects of administration of iron, iodine and simultaneous iron‐plus‐iodine on the thyroid hormone profile in iron‐deficient adolescent Iranian girls. European Journal of Clinical Nutrition 2006;60(4):545‐52. - PubMed
Elwood 1966 {published data only}
Elwood 1970 {published data only}
Flink 2006 {published data only}
-
- Flink H, Tegelberg A, Thörn M, Lagerlöf F. Effect of oral iron supplementation on unstimulated salivary flow rate: a randomized, double‐blind, placebo‐controlled trial. Journal of Oral Pathology & Medicine 2006;35(9):540‐7. [PUBMED: 16968234] - PubMed
Florencio 1981 {published data only}
-
- Florencio CA. Effects of iron and ascorbic acid supplementation on hemoglobin level and work efficiency of anemic women. Journal of Occupational and Environmental Medicine 1981;23(10):699‐704. - PubMed
Fogelholm 1992 {published data only}
-
- Fogelholm M, Jaakkola L, Lampisjärvi T. Effects of iron supplementation in female athletes with low serum ferritin concentration. International Journal of Sports Medicine 1992;13(2):158‐62. [PUBMED: 1555906 ] - PubMed
Fogelholm 1994 {published data only}
-
- Fogelholm M, Suominen M, Rita H. Effects of low‐dose iron supplementation in women with low serum ferritin concentration. European Journal of Clinical Nutrition 1994;48(10):753‐6. [PUBMED: 7835330] - PubMed
Gordeuk 1987 {published data only}
-
- Gordeuk VR, Brittenham GM, Hughes MA, Keating LJ. Carbonyl iron for short‐term supplementation in female blood donors. Transfusion 1987;27(1):80‐5. - PubMed
Gordeuk 1990 {published data only}
-
- Gordeuk VR, Brittenham GM, Bravo J, Hughes MA, Keating LJ. Prevention of iron deficiency with carbonyl iron in female blood donors. Transfusion 1990;30(3):239‐45. [PUBMED: 2180144] - PubMed
Gunaratna 2015 {published data only}
Heath 2001 {published data only}
-
- Heath AL, Skeaff CM, O'Brien SM, Williams SM, Gibson RS. Can dietary treatment of non‐anemic iron deficiency improve iron status?. Journal of the American College of Nutrition 2001;20(5):477‐84. [PUBMED: 11601562] - PubMed
Hinton 2000 {published data only}
-
- Brownlie T, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. American Journal of Clinical Nutrition 2002;75(4):734‐42. [PUBMED: 11916761] - PubMed
-
- Brownlie T, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. American Journal of Clinical Nutrition 2004;79(3):437‐43. [PUBMED: 14985219] - PubMed
-
- Hinton PS, Giordano C, Brownlie T, Haas JD. Iron supplementation improves endurance after training in iron‐depleted, nonanemic women. Journal of Applied Physiology 2000;88(3):1103‐11. [PUBMED: 10710409] - PubMed
Hinton 2007 {published data only}
Hoppe 2013 {published data only}
-
- Hoppe M, Brün B, Larsson MP, Moraeus L, Hulthén L. Heme iron‐based dietary intervention for improvement of iron status in young women. Nutrition 2013;29(1):89‐95. [PUBMED: 22951158] - PubMed
Jayatissa 1999 {published data only}
-
- Jayatissa R, Piyasena C. Adolescent schoolgirls: daily or weekly iron supplementation?. Food and Nutrition Bulletin 1999;20(4):429‐34.
Jensen 1991 {published data only}
-
- Jensen CA, Weaver CM, Sedlock DA. Iron supplementation and iron status in exercising young women. Journal of Nutritional Biochemistry 1991;2(7):368‐73. [DOI: 10.1016/0955-2863(91)90093-K] - DOI
Kanani 2000 {published data only}
-
- Kanani SJ, Poojara RH. Supplementation with iron and folic acid enhances growth in adolescent Indian girls. Journal of Nutrition 2000;130(2S Suppl):452S‐5S. - PubMed
Kang 2004 {published data only}
-
- Kang HS, Matsuo T. Effects of 4 weeks iron supplementation on haematological and immunological status in elite female soccer players. Asia Pacific Journal of Clinical Nutrition 2004;13(4):353‐8. [PUBMED: 15563440] - PubMed
Kianfar 2000 {published data only}
-
- Kianfar H, Kimiagar M, Ghaffarpour M. Effect of daily and intermittent iron supplementation on iron status of high school girls. International Journal for Vitamin and Nutrition Research 2000;70(4):172‐7. [PUBMED: 10989766] - PubMed
Kiss 2015 {published data only}
-
- Cable G, Brambilla D, Glynn S, Mast AE, Kiss JE. Quantification of iron stores, body iron, and iron absorption in blood donors: effect of iron supplementation. Transfusion. Abstract Presentations from the AABB Annual Meeting; 2014 October 25‐28; Philadelphia, (PA). 2014; Vol. 54 Suppl S2:37A.
Klingshirn 1992 {published data only}
-
- Klingshirn LA, Pate RR, Bourque SP, Davis JM, Sargent RG. Effect of iron supplementation on endurance capacity in iron‐depleted female runners. Medicine and Science in Sports and Exercise 1992;24(7):819‐24. - PubMed
LaManca 1993 {published data only}
-
- LaManca JJ. Effects of Iron Supplementation on Aerobic Power, Endurance Performance, Blood Lactate, and Body Iron Stores in Women [Post doctoral thesis]. Florida: Florida State University, 1989.
-
- LaManca JJ, Haymes EM. Effects of iron repletion on VO2max, endurance, and blood lactate in women. Medicine and Science in Sports and Exercise 1993;25(12):1386‐92. [PUBMED: 8107547] - PubMed
Lanerolle 2000 {published data only}
-
- Lanerolle P, Atukorala S, Silva G, Samarasinghe S, Dharmawardena L. Evaluation of nutrition education for improving iron status in combination with daily iron supplementation. Food and Nutrition Bulletin 2000;21(3):259‐69. [DOI: 10.1177/156482650002100302] - DOI
Larocque 2006 {published data only}
-
- Larocque T. Iron Status and Cognitive Performance in Adolescent Females [thesis]. Lakehead University, 2006.
Leonard 2014 {published data only}
-
- Leonard AJ. Iron Deficiency in Young Australian Women: Role of Iron Knowledge, Dietary Intake and Supplementation, and the Effects on Cognition [Thesis]. Newcastle Australia: University of Newcastle, 2014.
Li 1994 {published data only}
-
- Li R, Chen X, Yan H, Deurenberg P, Garby L, Hautvast JG. Functional consequences of iron supplementation in iron‐deficient female cotton mill workers in Beijing, China. American Journal of Clinical Nutrition 1994;59(4):908‐13. - PubMed
Lyle 1992 {published data only}
-
- Lyle RM, Weaver CM, Sedlock DA, Rajaram S, Martin B, Melby CL. Iron status in exercising women: the effect of oral iron therapy vs increased consumption of muscle foods. American Journal of Clinical Nutrition 1992;56(6):1049‐55. - PubMed
Machado 2011 {published data only}
-
- Machado KMM, Ferreira LOC, Souza AI, Diniz AS. The side‐effects of different doses of iron sulfate on women of reproductive age: a randomized double‐blind, placebo controlled study [Efeitos colaterais do sulfato ferroso administrado em diferentes posologias em mulheres em idade reprodutiva: estudo randomizado, duplo cego e controlado por placebo]. Revista Brasileira de Saúde Materno Infantil 2011;11(3):275‐81. [DOI: 10.1590/S1519-38292011000300008] - DOI
Magazanik 1991 {published data only}
-
- Magazanik A, Weinstein Y, Abarbanel J, Lewinski U, Shapiro Y, Inbar O, et al. Effect of an iron supplement on body iron status and aerobic capacity of young training women. European Journal of Applied Physiology and Occupational Physiology 1991;62(5):317‐23. - PubMed
Maghsudlu 2008 {published data only}
Marks 2014 {published and unpublished data}
-
- Marks DC, Speedy J, Robinson KL, Brama T, Capper HR, Mondy P, et al. An 8‐week course of 45 mg of carbonyl iron daily reduces iron deficiency in female whole blood donors aged 18 to 45 years: results of a prospective randomized controlled trial. Transfusion 2014;54(3 Pt 2):780‐8. [DOI: 10.1111/trf.12464] - DOI - PubMed
McClung 2009 {published data only}
-
- McClung JP, Karl JP, Cable SJ, Williams KW, Nindl BC, Young AJ, et al. Randomized, double‐blind, placebo‐controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. American Journal of Clinical Nutrition 2009;90(1):124‐31. [PUBMED: 19474138] - PubMed
Mujica‐Coopman 2015 {published data only}
-
- Mujica‐Coopman MF, Borja A, Pizarro F, Olivares M. Effect of daily supplementation with iron and zinc on iron status of childbearing age women. Biological Trace Element Research 2015;165(1):10‐7. - PubMed
Murray‐Kolb 2007 {published data only (unpublished sought but not used)}
-
- Murray‐Kolb LE. Iron Status, Cognitive Performance, and Behavioral Affect in Young Women [Doctoral thesis]. Pennsylvania State University, 2003.
-
- Murray‐Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. American Journal of Clinical Nutrition 2007;85(3):778‐87. [PUBMED: 17344500] - PubMed
Newhouse 1989 {published data only}
Pereira 2014 {published and unpublished data}
-
- Pereira, D. Question re your paper in BMC Gastroenterology 2014 [personal communication]. Email to: SR Pasricha 3 November 2014.
Prosser 2010 {published data only}
Radjen 2011 {published data only}
-
- Radjen S, Radjen G, Životić‐Vanović M, Radaković S, Vasiljević N, Stojanovic D. Effect of iron supplementation on maximal oxygen uptake in female athletes [Uticaj suplementacije gvožđa na maksimalnu potrošnju kiseonika kod sportistkinja]. Military‐Medical and Pharmaceutical Review 2011;68(2):130‐5. [DOI: 10.2298/VSP1102130R] - DOI - PubMed
Rajaram 1995 {published data only}
-
- Rajaram S, Weaver CM, Lyle RM, Sedlock DA, Martin B, Templin TJ, et al. Effects of long‐term moderate exercise on iron status in young women. Medicine and Science in Sports and Exercise 1995;27(8):1105‐10. - PubMed
Rowland 1988 {published data only}
Rybo 1985 {published data only}
Røsvik 2010 {published data only}
Shah 2002 {published data only}
-
- Shah BK, Gupta P. Weekly vs daily iron and folic acid supplementation in adolescent Nepalese girls. Archives of Pediatrics & Adolescent Medicine 2002; Vol. 156, issue 2:131‐5. [PUBMED: 11814373] - PubMed
Swain 2007 {published data only}
-
- Swain JH, Johnson LK, Hunt JR. Electrolytic iron or ferrous sulfate increase body iron in women with moderate to low iron stores. Journal of Nutrition 2007;137(3):620‐7. [PUBMED: 17311950] - PubMed
Taniguchi 1991 {published data only}
-
- Taniguchi M, Imamura H, Shirota T, Okamatsu H, Fujii Y, Toba M, et al. Improvement in iron deficiency anemia through therapy with ferric ammonium citrate and vitamin C and the effects of aerobic exercise. Journal of Nutritional Science and Vitaminology 1991; Vol. 37, issue 2:161‐71. [PUBMED: 1919803] - PubMed
Verdon 2003 {published data only}
Viteri 1999 {published data only}
-
- Viteri FE, Ali F, Tujague J. Long‐term weekly iron supplementation improves and sustains nonpregnant women's iron status as well or better than currently recommended short‐term daily supplementation. Journal of Nutrition 1999;129(11):2013‐20. - PubMed
Waldvogel 2012 {published data only}
Walsh 1989 {published data only (unpublished sought but not used)}
-
- Walsh R, McNaughton L. Effects of iron supplementation on iron status of young female swimmers during pre‐season phase of competition. Journal of Swimming Research 1989;5(2):13‐8.
Wang 2012 {published data only}
-
- Wang Z, Sun J, Wang L, Zong M, Chen Y, Lin Y, et al. Effect of iron supplementation on iron deficiency anemia of childbearing age women in Shanghai. Journal of Hygiene Research 2012;41(1):51‐5. [PUBMED: 22443058] - PubMed
Yadrick 1989 {published data only}
-
- Yadrick MK, Kenney MA, Winterfeldt EA. Iron, copper, and zinc status: response to supplementation with zinc or zinc and iron in adult females. American Journal of Clinical Nutrition 1989;49(1):145‐50. [PUBMED: 2912000] - PubMed
Yoshida 1990 {published data only}
-
- Yoshida T, Udo M, Chida M, Ichioka M, Makiguchi K. Dietary iron supplement during severe physical training in competitive female distance runners. Sports Training, Medicine and Rehabilitation 1990;1(4):279‐85. [DOI: 10.1080/15438629009511885] - DOI
Zaman 2013 {published data only}
-
- McArthur JO, Petocz P, Caterson ID, Samman S. A randomized controlled trial in young women of the effects of consuming pork meat or iron supplements on nutritional status and feeling of well‐being. Journal of the American College of Nutrition 2012;31(3):175‐84. [DOI: 10.1080/07315724.2012.10720025] - DOI - PubMed
Zavaleta 2000 {published data only}
-
- Zavaleta N, Respicio G, Garcia T. Efficacy and acceptability of two iron supplementation schedules in adolescent school girls in Lima, Peru. Journal of Nutrition 2000;130(2):462S‐4S. - PubMed
Zhu 1998 {published data only}
-
- Zhu YI, Haas JD. Altered metabolic response of iron‐depleted nonanemic women during a 15‐km time trial. Journal of Applied Physiology 1998 May;84(5):1768‐75. - PubMed
-
- Zhu YI, Haas JD. Response of serum transferrin receptor to iron supplementation in iron‐depleted, nonanemic women. American Journal of Clinical Nutrition 1988;67(2):271‐5. - PubMed
References to studies excluded from this review
Brigham 1993 {published data only}
-
- Brigham DE, Beard JL, Krimmel RS, Kenney WL. Changes in iron status during competitive season in female collegiate swimmers. Nutrition 1993;9(5):418‐22. [PUBMED: 8286880] - PubMed
Cable 1988 {published data only}
Powell 1991 {published data only}
-
- Powell PD, Tucker A. Iron supplementation and running performance in female cross‐country runners. International Journal of Sports Medicine 1991;12(5):462‐7. - PubMed
Powers 1988 {published data only}
-
- Powers HJ, Bates CJ, Downes R, Brubacher D, Sutcliffe V, Thurnhill A. Running performance in Gambian children: effects of water‐soluble vitamins on iron. European Journal of Clinical Nutrition 1988;42(11):895‐902. - PubMed
Schoene 1983 {published data only}
-
- Schoene RB, Escourrou P, Robertson HT, Nilson KL, Parsons JR, Smith NJ. Iron repletion decreases maximal exercise lactate concentrations in female athletes with minimal iron‐deficiency anemia. Journal of Laboratory and Clinical Medicine 1983;102(2):306‐12. [PUBMED: 6864076] - PubMed
Simon 1984 {published data only}
-
- Simon TL, Hunt WC, Garry PJ. Iron supplementation for menstruating female blood donors. Transfusion 1984;24(6):469‐72. [PUBMED: 6506175] - PubMed
References to studies awaiting assessment
Blot 1980 {published data only}
-
- Blot I, Chenayer M, Diakhate L, Leluc R, Gross E, Tchernia G. Iron reserves in blood donors. Should we prescribe systematic iron supplementation?. Revue Francaise de Transfusion et Immuno‐hematologie 1980;23(2):119‐29. - PubMed
Böttiger 1971 {published data only}
-
- Böttiger LE, Nyberg A, Astrand I, Astrand PO. Iron administration to healthy, physically very active students. Nordisk Medicin 1971;85(13):396‐8. [PUBMED: 4928926] - PubMed
Charoenlarp 1981 {published data only}
-
- Charoenlarp P. Iron supplementation studies. Experimental design and interpretation of results. Progress in Clinical and Biological Research 1981;77:355‐61. - PubMed
Greene 1995 {published data only}
-
- Greene LM. Effect of Iron Supplementation on Measures of Cognitive Functioning During Adolescence. [Thesis]. University of Ulster, 1995.
Isager 1974 {published data only}
-
- Isager H. Iron deficiency, growth, and stimulated erythropoiesis. Scandinavian Journal of Haematology. Supplementum 1974;21:1‐176. - PubMed
Izak 1973 {published data only}
-
- Izak G, Levy S, Rachmilewitz M, Grossowicz N. The effect of iron and folic acid therapy on combined iron and folate deficiency anaemia: the results of a clinical trial. Scandinavian Journal of Haematology 1973;11(3):236‐40. - PubMed
Parkinson 1981 {published data only}
-
- Parkinson CF, Parkinson SB. Effect of vitamin‐iron preparation on tooth staining studied. Journal of the Missouri Dental Association 1981;61(4):34‐7. - PubMed
References to ongoing studies
IRCT201409082365N9 {published data only}
-
- IRCT201409082365N9. The effects of vitamin D or iron‐vitamin supplementation on bone metabolism and inflammation in 18‐40‐year women. http://apps.who.int/trialsearch/Trial2.aspx?trialid=IRCT201409082365N9 (accessed 15 November 2015).
Additional references
Andrews 1999
Balshem 2011
Borenstein 2008
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice). Chichester, UK: John Wiley & Sons, 2008.
Brainclinics 2015 [Computer program]
-
- Brainclinics Products. Neuropsychological tests: IntegNeuro. Nijmegen, The Netherlands: Brainclinics Product, 2015.
Brownlie 2002
-
- Brownlie T, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. American Journal of Clinical Nutrition 2002;75(4):734‐42. - PubMed
Brownlie 2004
-
- Brownlie T 4th, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. American Journal of Clinical Nutrition 2004;79(3):437‐43. - PubMed
Burhans 2005
-
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray‐Kolb LJ, Byron C, et al. Iron deficiency: differential effects on monoamine transporters. Nutritional Neuroscience 2005;8(1):31‐8. - PubMed
Chambers 2009
Dallman 1992
-
- Dallman PR. Changing iron needs from birth through adolescence. In: Fomon SJ, Zlotkin S editor(s). Nutritional Anemias. Vol. 30, New York: Nestle Nutrition Workshop Series, Nestec Ltd. Vevey/Raven Press Ltd, 1992:34.
De‐Regil 2011
Deeks 2011
-
- Deeks J, Higgins JPT, Altman D. Chapter 9: Analysing data and undertaking meta‐analyses. In Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dodd 2004
Endnote 2015 [Computer program]
-
- McCracken MO. Endnote X7. Thomson Reuters, 2015.
Fernández‐Gaxiola 2011
Gandek 1998
-
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross‐validation of item selection and scoring for the SF‐12 Health Survey in nine countries: results from the IQOLA Project. Journal of Clinical Epidemiology 1998;51(11):1171‐8. [DOI: 10.1016/S0895-4356(98)00109-7] - DOI - PubMed
Goddard 2011
GRADEpro GDT 2015 [Computer program]
-
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University, 2015 (developed by Evidence Prime, Inc.) Available from www.gradepro.org.
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876‐83. [PUBMED: 10221325] - PubMed
Haider 2006
Harbord 2009
-
- Harbord RM, Harris RJ, Sterne JAC. Updated tests for small–study effects in meta–analyses. The Stata Journal 2009;9(2):197‐210.
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hotez 2005
Hurrell 2010
IOC 2009
-
- International Olympic Committee. The International Olympic Committee (IOC) consensus statement on periodic health evaluation of elite athletes: March 2009. http://bit.ly/1Wn3aJX (accessed 25 January 2015).
Krupp 1989
-
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46(10):1121‐3. [PUBMED: 2803071] - PubMed
Low 2013
Lozoff 2007
-
- Lozoff B. Iron deficiency and child development. Food and Nutrition Bulletin 2007;28(4 Suppl):S560‐71. - PubMed
Mahoney 2011
-
- Mahoney DH. Iron deficiency in infants and young children: treatment. www.uptodate.com/contents/iron‐deficiency‐in‐infants‐and‐young‐children‐... (accessed 31 August 2011).
McNair 1971
-
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service, 1971.
Okebe 2011
Oppenheimer 2001
-
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131(2):S616‐35S. - PubMed
Pasricha 2010
-
- Pasricha SR, Flecknoe‐Brown SC, Allen KJ, Gibson PR, McMahon LP, Olynyk JK, et al. Diagnosis and management of iron deficiency anaemia: a clinical update. Medical Journal of Australia 2010;193(9):525‐32. - PubMed
Pasricha 2013
-
- Pasricha SR, Hayes E, Kalumba K, Biggs BA. Effect of daily iron supplementation on health in children aged 4‐23 months: a systematic review and meta‐analysis of randomised controlled trials. Lancet Global Health 2013;1(2):e77‐86. [PUBMED: 25104162 ] - PubMed
Pasrischa 2012
-
- Pasricha S‐R, De‐Regil LM, Garcia‐Casal MN, Burford BJ, Gwirtz JA, Peña‐Rosas JP. Fortification of maize flour with iron for preventing anaemia and iron deficiency in populations. Cochrane Database of Systematic Reviews 14 November 2012;11:Art. No.: CD010187. DOI: 10.1002/14651858.CD010187. - PMC - PubMed
Peeling 2008
-
- Peeling P, Dawson B, Goodman C, Landers G, Trinder D. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. European Journal of Applied Physiology 2008;103(4):381‐94. - PubMed
Peña‐Rosas 2009
Peña‐Rosas 2014
Radloff 1977
-
- Radloff LS. The CES‐D Scale. A self‐report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385‐401. [DOI: 10.1177/014662167700100306] - DOI
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scholz 1997
Self 2012
Sharpe 1950
-
- Sharpe LM, Peacock WC, Cooke R, Harris RS. The effect of phytate and other food factors on iron absorption. Journal of Nutrition 1950;41(3):433‐46. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stevens 2013
-
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995–2011: a systematic analysis of population‐representative data. Lancet Global Health 2013;1(1):e16‐25. [DOI: 10.1016/S2214-109X(13)70001-9] - DOI - PMC - PubMed
Stoltzfus 2001
-
- Stoltzfus R. Defining iron‐deficiency anemia in public health terms: a time for reflection. Journal of Nutrition 2001;131(2S‐2):565S‐7S. - PubMed
Suominen 1998
-
- Suominen P, Punnonen K, Rajamäki A, Irjala K. Serum transferrin receptor and transferrin receptor‐ferritin index identify healthy subjects with subclinical iron deficits. Blood 1998;92(8):2934. - PubMed
Thankachan 2008
-
- Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. American Journal of Clinical Nutrition 2008;87(4):881‐6. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):i‐97. [PUBMED: 10982317] - PubMed
Umbreit 2005
Ware 1992
-
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] - PubMed
WHO 2009
-
- World Health Organization. Weekly iron–folic acid supplementation (WIFS) in women of reproductive age: its role in promoting optimal maternal and child health. www.who.int/nutrition/publications/micronutrients/weekly_iron_folicacid/... (accessed 31 August 2011). - PubMed
WHO 2010
-
- World Health Organization. World Malaria Report 2010. http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf (accessed 31 August 2011).
WHO 2011
-
- World Health Organization. Prevention of Iron Deficiency Anaemia in Adolescents. Role of Weekly Iron and Folic Acid Supplementation. 1st Edition. Vol. 1, New Delhi: SEARO, World Health Organization, 2011.
WHO/CDC 2008
-
- World Health Organization/Centers for Disease Control and Prevention. Worldwide prevalence of anaemia 1993‐2005: WHO global database on anaemia. http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf (accessed 31 August 2011).
WHO/UNICEF/UNU 2001
-
- World Health Organization/United Nations Children's Fund/United Nations University. Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. Geneva: World Health Organization, 2001.
Wolgemuth 1982
-
- Wolgemuth JC, Latham MC, Hall A, Chesher A, Crompton DW. Worker productivity and the nutritional status of Kenyan road construction laborers. American Journal of Clinical Nutrition 1982;36(1):68‐78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

