Reduced costs of reproduction in females mediate a shift from a male-biased to a female-biased lifespan in humans
- PMID: 27087670
- PMCID: PMC4834564
- DOI: 10.1038/srep24672
Reduced costs of reproduction in females mediate a shift from a male-biased to a female-biased lifespan in humans
Abstract
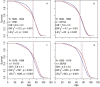
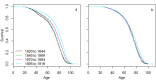
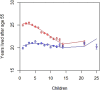
The causes underlying sex differences in lifespan are strongly debated. While females commonly outlive males in humans, this is generally less pronounced in societies before the demographic transition to low mortality and fertility rates. Life-history theory suggests that reduced reproduction should benefit female lifespan when females pay higher costs of reproduction than males. Using unique longitudinal demographic records on 140,600 reproducing individuals from the Utah Population Database, we demonstrate a shift from male-biased to female-biased adult lifespans in individuals born before versus during the demographic transition. Only women paid a cost of reproduction in terms of shortened post-reproductive lifespan at high parities. Therefore, as fertility decreased over time, female lifespan increased, while male lifespan remained largely stable, supporting the theory that differential costs of reproduction in the two sexes result in the shifting patterns of sex differences in lifespan across human populations. Further, our results have important implications for demographic forecasts in human populations and advance our understanding of lifespan evolution.
Figures
References
-
- Trivers R. L. In Sexual selection and the descent of man (ed Campbell B. G.) 136–207 (Aldine, 1972).
-
- Williams G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411, doi: 10.2307/2406060 (1957). - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

