Early-onset obesity dysregulates pulmonary adipocytokine/insulin signaling and induces asthma-like disease in mice
- PMID: 27087690
- PMCID: PMC4834579
- DOI: 10.1038/srep24168
Early-onset obesity dysregulates pulmonary adipocytokine/insulin signaling and induces asthma-like disease in mice
Abstract
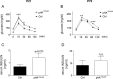
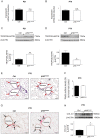
Childhood obesity is a risk factor for asthma, but the molecular mechanisms linking both remain elusive. Since obesity leads to chronic low-grade inflammation and affects metabolic signaling we hypothesized that postnatal hyperalimentation (pHA) induced by maternal high-fat-diet during lactation leads to early-onset obesity and dysregulates pulmonary adipocytokine/insulin signaling, resulting in metabolic programming of asthma-like disease in adult mice. Offspring with pHA showed at postnatal day 21 (P21): (1) early-onset obesity, greater fat-mass, increased expression of IL-1β, IL-23, and Tnf-α, greater serum leptin and reduced glucose tolerance than Control (Ctrl); (2) less STAT3/AMPKα-activation, greater SOCS3 expression and reduced AKT/GSK3β-activation in the lung, indicative of leptin resistance and insulin signaling, respectively; (3) increased lung mRNA of IL-6, IL-13, IL-17A and Tnf-α. At P70 body weight, fat-mass, and cytokine mRNA expression were similar in the pHA and Ctrl, but serum leptin and IL-6 were greater, and insulin signaling and glucose tolerance impaired. Peribronchial elastic fiber content, bronchial smooth muscle layer, and deposition of connective tissue were not different after pHA. Despite unaltered bronchial structure mice after pHA exhibited significantly increased airway reactivity. Our study does not only demonstrate that early-onset obesity transiently activates pulmonary adipocytokine/insulin signaling and induces airway hyperreactivity in mice, but also provides new insights into metabolic programming of childhood obesity-related asthma.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

