Ecology and evolution of pathogens in natural populations of Lepidoptera
- PMID: 27087850
- PMCID: PMC4780379
- DOI: 10.1111/eva.12328
Ecology and evolution of pathogens in natural populations of Lepidoptera
Abstract
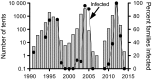
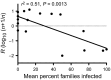
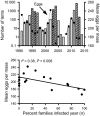
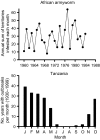
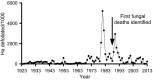
Pathogens are ubiquitous in insect populations and yet few studies examine their dynamics and impacts on host populations. We discuss four lepidopteran systems and explore their contributions to disease ecology and evolution. More specifically, we elucidate the role of pathogens in insect population dynamics. For three species, western tent caterpillars, African armyworm and introduced populations of gypsy moth, infection by nucleopolyhedrovirus (NPV) clearly regulates host populations or reduces their outbreaks. Transmission of NPV is largely horizontal although low levels of vertical transmission occur, and high levels of covert infection in some cases suggest that the virus can persist in a nonsymptomatic form. The prevalence of a mostly vertically transmitted protozoan parasite, Ophryocystis elektroscirrha, in monarch butterflies is intimately related to their migratory behaviour that culls highly infected individuals. Virulence and transmission are positively related among genotypes of this parasite. These systems clearly demonstrate that the interactions between insects and pathogens are highly context dependent. Not only is the outcome a consequence of changes in density and genetic diversity: environmental factors, particularly diet, can have strong impacts on virulence, transmission and host resistance or tolerance. What maintains the high level of host and pathogen diversity in these systems, however, remains a question.
Keywords: disease ecology; disease transmission; forest Lepidoptera; insect pathogens; migration; population regulation; tritrophic interactions; virulence.
Figures


References
-
- Allstadt, A. J. , Haynes K. J., Liebhold A. M., and Johnson D. M. 2013. Long‐term shifts in the cyclicity of outbreaks of a forest‐defoliating insect. Oecologia 172:141–151. - PubMed
-
- Altizer, S. M. , and Oberhauser K. S. 1999. Effects of the protozoan parasite Ophryocystis elektroscirrha on the fitness of monarch butterflies (Danaus plexippus). Journal of Invertebrate Pathology 74:76–88. - PubMed
-
- Altizer, S. M. , Oberhauser K. S., and Brower L. P. 2000. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecological Entomology 25:125–139.
-
- Altizer, S. , Bartel R., and Han B. A. 2011. Animal migration and infectious disease risk. Science 331:296–302. - PubMed
-
- Alvarado‐Rodriguez, B. 1987. Parasites and disease associated with larvae of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae), infesting processing tomatoes in Sinaloa, Mexico. Florida Entomologist 70:444–449.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

