A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments
- PMID: 27087910
- PMCID: PMC4821453
- DOI: 10.1016/j.csbj.2016.02.005
A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments
Abstract
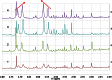
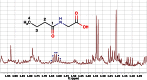
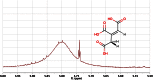
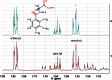
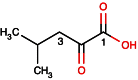
Metabonomics/metabolomics is an important science for the understanding of biological systems and the prediction of their behaviour, through the profiling of metabolites. Two technologies are routinely used in order to analyse metabolite profiles in biological fluids: nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS), the latter typically with hyphenation to a chromatography system such as liquid chromatography (LC), in a configuration known as LC-MS. With both NMR and MS-based detection technologies, the identification of the metabolites in the biological sample remains a significant obstacle and bottleneck. This article provides guidance on methods for metabolite identification in biological fluids using NMR spectroscopy, and is illustrated with examples from recent studies on mice.
Keywords: Metabolite identification; Metabolomics; Metabonomics; Molecular structure; Nuclear magnetic resonance (NMR) spectroscopy.
Figures


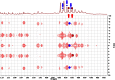
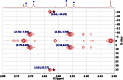
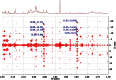
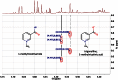
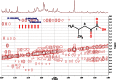
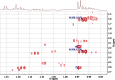
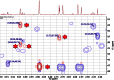
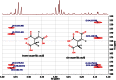
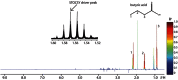
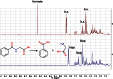
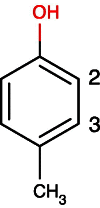
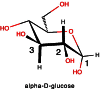
References
-
- Lindon J., Nicholson J., Holmes E., Everett J. Metabonomics: metabolic processes studied by NMR spectroscopy of biofluids. Concepts Magn Reson. 2000;12(5):289–320.
-
- Fiehn O. Metabolomics—the link between genotypes and phenotypes. Plant Mol Biol. 2002;48(1–2):155–171. - PubMed
-
- Lindon J.C., Nicholson J.K., Holmes E. Elsevier; Amsterdam, Oxford: 2007. The Handbook of Metabonomics and Metabolomics.
-
- Clayton T., Lindon J., Cloarec O., et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature. 2006;440(7087):1073–1077. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
