Glioblastoma: Defining Tumor Niches
- PMID: 27088132
- PMCID: PMC4831073
- DOI: 10.1016/j.trecan.2015.10.009
Glioblastoma: Defining Tumor Niches
Abstract
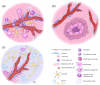
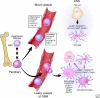
Glioblastomas (GBM) are one of the most recalcitrant brain tumors because of their aggressive invasive growth and resistance to therapy. They are highly heterogeneous malignancies at both the molecular and histological levels. Specific histological hallmarks including pseudopalisading necrosis and microvascular proliferation distinguish GBM from lower-grade gliomas, and make GBM one of the most hypoxic as well as angiogenic tumors. These microanatomical compartments present specific niches within the tumor microenvironment that regulate metabolic needs, immune surveillance, survival, invasion as well as cancer stem cell maintenance. Here we review features and functions of the distinct GBM niches, detail the different cell constituents and the functional status of the vasculature, and discuss prospects of therapeutically targeting GBM niche constituents.
Keywords: GBM; astrocytes; cancer stem cells; macrophages; microglial cells; therapy; tumor microenvironment; tumor niches; tumor vasculature.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
