Expansion of Shiga Toxin-Producing Escherichia coli by Use of Bovine Antibiotic Growth Promoters
- PMID: 27088186
- PMCID: PMC4861518
- DOI: 10.3201/eid2205.151584
Expansion of Shiga Toxin-Producing Escherichia coli by Use of Bovine Antibiotic Growth Promoters
Abstract
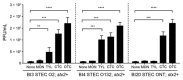
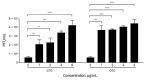
Antibiotics are routinely used in food-producing animals to promote growth and prevent infectious diseases. We investigated the effects of bovine antibiotic growth promoters (bAGPs) on the propagation and spread of Shiga toxin (Stx)-encoding phages in Escherichia coli. Co-culture of E. coli O157:H7 and other E. coli isolated from cattle in the presence of sublethal concentrations of bAGPs significantly increased the emergence of non-O157, Stx-producing E. coli by triggering the SOS response system in E. coli O157:H7. The most substantial mediation of Stx phage transmission was induced by oxytetracyline and chlortetracycline, which are commonly used in agriculture. bAGPs may therefore contribute to the expansion of pathogenic Stx-producing E. coli.
Keywords: E. coli; STEC; Shiga toxin–producing Escherichia coli; Stx-encoding bacteriophages; antimicrobial resistance; bacteria; bovine antibiotic growth promoters.
Figures
Similar articles
-
Phage applications for biocontrol of enterohemorrhagic E. coli O157:H7 and other Shiga toxin-producing Escherichia coli.Int J Food Microbiol. 2025 Aug 2;439:111267. doi: 10.1016/j.ijfoodmicro.2025.111267. Epub 2025 May 13. Int J Food Microbiol. 2025. PMID: 40382813 Review.
-
Shiga toxin-producing Escherichia coli strains from cattle as a source of the Stx2a bacteriophages present in enteroaggregative Escherichia coli O104:H4 strains.Int J Med Microbiol. 2013 Dec;303(8):595-602. doi: 10.1016/j.ijmm.2013.08.001. Epub 2013 Aug 16. Int J Med Microbiol. 2013. PMID: 24012149
-
Characterization of a T4-like Bacteriophage vB_EcoM-Sa45lw as a Potential Biocontrol Agent for Shiga Toxin-Producing Escherichia coli O45 Contaminated on Mung Bean Seeds.Microbiol Spectr. 2022 Feb 23;10(1):e0222021. doi: 10.1128/spectrum.02220-21. Epub 2022 Feb 2. Microbiol Spectr. 2022. PMID: 35107386 Free PMC article.
-
Comparable stx2a expression and phage production levels between Shiga toxin-producing Escherichia coli strains from human and bovine origin.Zoonoses Public Health. 2020 Feb;67(1):44-53. doi: 10.1111/zph.12653. Epub 2019 Dec 23. Zoonoses Public Health. 2020. PMID: 31868306
-
Implications of free Shiga toxin-converting bacteriophages occurring outside bacteria for the evolution and the detection of Shiga toxin-producing Escherichia coli.Front Cell Infect Microbiol. 2014 Apr 16;4:46. doi: 10.3389/fcimb.2014.00046. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24795866 Free PMC article. Review.
Cited by
-
Pathogenic Factors and Recent Study on the Rapid Detection of Shiga Toxin-Producing Escherichia coli (STEC).Mol Biotechnol. 2025 Jan;67(1):16-26. doi: 10.1007/s12033-023-00985-8. Epub 2023 Dec 28. Mol Biotechnol. 2025. PMID: 38153662 Review.
-
Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review.Toxins (Basel). 2020 Jan 21;12(2):67. doi: 10.3390/toxins12020067. Toxins (Basel). 2020. PMID: 31973203 Free PMC article. Review.
-
Zinc blocks SOS-induced antibiotic resistance via inhibition of RecA in Escherichia coli.PLoS One. 2017 May 22;12(5):e0178303. doi: 10.1371/journal.pone.0178303. eCollection 2017. PLoS One. 2017. PMID: 28542496 Free PMC article.
-
Prevalence and Implications of Shiga Toxin-Producing E. coli in Farm and Wild Ruminants.Pathogens. 2022 Nov 11;11(11):1332. doi: 10.3390/pathogens11111332. Pathogens. 2022. PMID: 36422584 Free PMC article. Review.
-
Occurrence, virulence genes, and antimicrobial profiles of Escherichia coli O157 isolated from ruminants slaughtered in Al Ain, United Arab Emirates.BMC Microbiol. 2020 Jul 16;20(1):210. doi: 10.1186/s12866-020-01899-0. BMC Microbiol. 2020. PMID: 32677884 Free PMC article.
References
-
- Organisation for Economic Co-operation Development. Global antimicrobial use in the livestock sector. 2015. [cited 2015 Jul 1]. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=TAD...
-
- Union of Concerned Scientists. Hogging it: estimates of antimicrobial abuse in livestock. 2001. [cited 2015 Jul 1]. http://www.ucsusa.org/food_and_agriculture/our-failing-food-system/indus...
-
- United States Department of Agriculture. Feedlot 2011 part IV: health and health management on U.S. feedlots with a capacity of 1,000 or more head. 2013. [cited 2015 Jul 1]. https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot...
-
- Giguère S, Prescott JF, Dowling PM. Antimicrobial therapy in veterinary medicine. 5th ed. Ames (IA): Wiley-Blackwell; 2013. p. 495–518.
-
- Nagaraja TG, Chengappa MM. Liver abscesses in feedlot cattle: a review. J Anim Sci. 1998;76:287–98 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

