Characterization of the FtsZ C-Terminal Variable (CTV) Region in Z-Ring Assembly and Interaction with the Z-Ring Stabilizer ZapD in E. coli Cytokinesis
- PMID: 27088231
- PMCID: PMC4835091
- DOI: 10.1371/journal.pone.0153337
Characterization of the FtsZ C-Terminal Variable (CTV) Region in Z-Ring Assembly and Interaction with the Z-Ring Stabilizer ZapD in E. coli Cytokinesis
Abstract
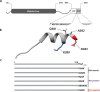
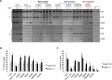
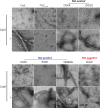
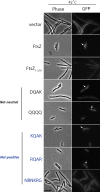
Polymerization of a ring-like cytoskeletal structure, the Z-ring, at midcell is a highly conserved feature in virtually all bacteria. The Z-ring is composed of short protofilaments of the tubulin homolog FtsZ, randomly arranged and held together through lateral interactions. In vitro, lateral associations between FtsZ protofilaments are stabilized by crowding agents, high concentrations of divalent cations, or in some cases, low pH. In vivo, the last 4-10 amino acid residues at the C-terminus of FtsZ (the C-terminal variable region, CTV) have been implicated in mediating lateral associations between FtsZ protofilaments through charge shielding. Multiple Z-ring associated proteins (Zaps), also promote lateral interactions between FtsZ protofilaments to stabilize the FtsZ ring in vivo. Here we characterize the complementary role/s of the CTV of E. coli FtsZ and the FtsZ-ring stabilizing protein ZapD, in FtsZ assembly. We show that the net charge of the FtsZ CTV not only affects FtsZ protofilament bundling, confirming earlier observations, but likely also the length of the FtsZ protofilaments in vitro. The CTV residues also have important consequences for Z-ring assembly and interaction with ZapD in the cell. ZapD requires the FtsZ CTV region for interaction with FtsZ in vitro and for localization to midcell in vivo. Our data suggest a mechanism in which the CTV residues, particularly K380, facilitate a conformation for the conserved carboxy-terminal residues in FtsZ, that lie immediately N-terminal to the CTV, to enable optimal contact with ZapD. Further, phylogenetic analyses suggest a correlation between the nature of FtsZ CTV residues and the presence of ZapD in the β- γ-proteobacterial species.
Conflict of interest statement
Figures

Similar articles
-
Structural and Biochemical Studies Reveal a Putative FtsZ Recognition Site on the Z-ring Stabilizer ZapD.Mol Cells. 2016 Nov 30;39(11):814-820. doi: 10.14348/molcells.2016.0202. Epub 2016 Nov 18. Mol Cells. 2016. PMID: 27871169 Free PMC article.
-
Structure of the Z Ring-associated Protein, ZapD, Bound to the C-terminal Domain of the Tubulin-like Protein, FtsZ, Suggests Mechanism of Z Ring Stabilization through FtsZ Cross-linking.J Biol Chem. 2017 Mar 3;292(9):3740-3750. doi: 10.1074/jbc.M116.773192. Epub 2017 Jan 18. J Biol Chem. 2017. PMID: 28100778 Free PMC article.
-
Structure and Mutational Analyses of Escherichia coli ZapD Reveal Charged Residues Involved in FtsZ Filament Bundling.J Bacteriol. 2016 May 13;198(11):1683-1693. doi: 10.1128/JB.00969-15. Print 2016 Jun 1. J Bacteriol. 2016. PMID: 27021560 Free PMC article.
-
Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring.Annu Rev Biochem. 2007;76:539-62. doi: 10.1146/annurev.biochem.75.103004.142652. Annu Rev Biochem. 2007. PMID: 17328675 Review.
-
FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one.Microbiol Mol Biol Rev. 2010 Dec;74(4):504-28. doi: 10.1128/MMBR.00021-10. Microbiol Mol Biol Rev. 2010. PMID: 21119015 Free PMC article. Review.
Cited by
-
Structural and Biochemical Studies Reveal a Putative FtsZ Recognition Site on the Z-ring Stabilizer ZapD.Mol Cells. 2016 Nov 30;39(11):814-820. doi: 10.14348/molcells.2016.0202. Epub 2016 Nov 18. Mol Cells. 2016. PMID: 27871169 Free PMC article.
-
Conserved Dynamics of Chloroplast Cytoskeletal FtsZ Proteins Across Photosynthetic Lineages.Plant Physiol. 2018 Jan;176(1):295-306. doi: 10.1104/pp.17.00558. Epub 2017 Aug 16. Plant Physiol. 2018. PMID: 28814573 Free PMC article.
-
Localization, Assembly, and Activation of the Escherichia coli Cell Division Machinery.EcoSal Plus. 2021 Dec 15;9(2):eESP00222021. doi: 10.1128/ecosalplus.ESP-0022-2021. Epub 2021 Dec 13. EcoSal Plus. 2021. PMID: 34910577 Free PMC article. Review.
-
Regulation of cytokinesis: FtsZ and its accessory proteins.Curr Genet. 2020 Feb;66(1):43-49. doi: 10.1007/s00294-019-01005-6. Epub 2019 Jun 17. Curr Genet. 2020. PMID: 31209564 Review.
-
Structure of the Z Ring-associated Protein, ZapD, Bound to the C-terminal Domain of the Tubulin-like Protein, FtsZ, Suggests Mechanism of Z Ring Stabilization through FtsZ Cross-linking.J Biol Chem. 2017 Mar 3;292(9):3740-3750. doi: 10.1074/jbc.M116.773192. Epub 2017 Jan 18. J Biol Chem. 2017. PMID: 28100778 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

