Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex
- PMID: 27088236
- PMCID: PMC4919903
- DOI: 10.1038/nmat4624
Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex
Abstract
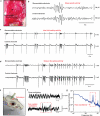
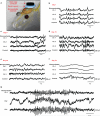
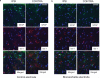
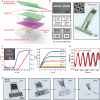
Bioresorbable silicon electronics technology offers unprecedented opportunities to deploy advanced implantable monitoring systems that eliminate risks, cost and discomfort associated with surgical extraction. Applications include postoperative monitoring and transient physiologic recording after percutaneous or minimally invasive placement of vascular, cardiac, orthopaedic, neural or other devices. We present an embodiment of these materials in both passive and actively addressed arrays of bioresorbable silicon electrodes with multiplexing capabilities, which record in vivo electrophysiological signals from the cortical surface and the subgaleal space. The devices detect normal physiologic and epileptiform activity, both in acute and chronic recordings. Comparative studies show sensor performance comparable to standard clinical systems and reduced tissue reactivity relative to conventional clinical electrocorticography (ECoG) electrodes. This technology offers general applicability in neural interfaces, with additional potential utility in treatment of disorders where transient monitoring and modulation of physiologic function, implant integrity and tissue recovery or regeneration are required.
Figures

References
-
- Niedermeyer E, da Silva FL. Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; 2005.
-
- McKhann GM, Schoenfeld-McNeill J, Born DE, Haglund MM, Ojemann GA. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. Journal of neurosurgery. 2000;93:44–52. - PubMed
-
- Litt B, et al. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron. 2001;30:51–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

