Pneumococcal Vaccination Strategies. An Update and Perspective
- PMID: 27088424
- PMCID: PMC5461988
- DOI: 10.1513/AnnalsATS.201511-778FR
Pneumococcal Vaccination Strategies. An Update and Perspective
Abstract
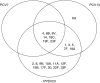
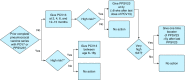
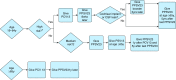
Streptococcus pneumoniae is an important global pathogen that causes a wide range of clinical disease in children and adults. Pneumococcal pneumonia is by far the common presentation of noninvasive and invasive pneumococcal disease and affects the young, the elderly, and the immunocompromised disproportionately. Patients with chronic pulmonary diseases are also at higher risk for pneumococcal infections. Substantial progress over the century has been made in the understanding of pneumococcal immunobiology and the prevention of invasive pneumococcal disease through vaccination. Currently, two pneumococcal vaccines are available for individuals at risk of pneumococcal disease: the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal protein-conjugate vaccine (PCV13). The goal of pneumococcal vaccination is to stimulate effective antipneumococcal antibody and mucosal immunity response and immunological memory. Vaccination of infants and young children with pneumococcal conjugate vaccine has led to significant decrease in nasal carriage rates and pneumococcal disease in all age groups. Recent pneumococcal vaccine indication and schedule recommendations on the basis of age and risk factors are outlined in this Focused Review. As new pneumococcal vaccine recommendations are being followed, continued efforts are needed to address the vaccine efficacy in the waning immunity of the ever-aging population, the implementation of vaccines using two different vaccines under very specific schedules and their real world clinical and cost effectiveness, and the development of next generation pneumococcal vaccines.
Keywords: PCV-13 vaccine; Streptococcus pneumoniae; pneumococcal vaccines; pneumonia.
Figures


References
-
- Atkinson W, Wolfe C, Hamborsky J, editors. Epidemiology and prevention of vaccine-preventable diseases. 12th ed. Washington DC: Public Health Foundation; 2012.
-
- Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–3412. - PubMed
-
- Austrian R. The pneumococcus at the millennium: not down, not out. J Infect Dis. 1999;179:S338–S341. - PubMed
-
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) report Emerging Infections Program Network Streptococcus pneumoniae; 2013 [accessed 2013 Dec 6]. Available from: http://www.cdc.gov/abcs/reports-findings/survreports/spneu12.pdf.
-
- van der Hoek W, Wielders CC, Schimmer B, Wegdam-Blans MC, Meekelenkamp J, Zaaijer HL, Schneeberger PM. Detection of phase I IgG antibodies to Coxiella burnetii with EIA as a screening test for blood donations. Eur J Clin Microbiol Infect Dis. 2012;31:3207–3209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

