Structure-Activity Relationship for the 4(3H)-Quinazolinone Antibacterials
- PMID: 27088777
- PMCID: PMC4885108
- DOI: 10.1021/acs.jmedchem.6b00372
Structure-Activity Relationship for the 4(3H)-Quinazolinone Antibacterials
Abstract
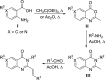
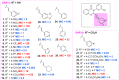
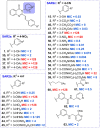
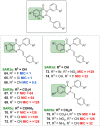
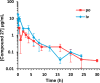
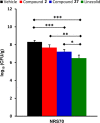
We recently reported on the discovery of a novel antibacterial (2) with a 4(3H)-quinazolinone core. This discovery was made by in silico screening of 1.2 million compounds for binding to a penicillin-binding protein and the subsequent demonstration of antibacterial activity against Staphylococcus aureus. The first structure-activity relationship for this antibacterial scaffold is explored in this report with evaluation of 77 variants of the structural class. Eleven promising compounds were further evaluated for in vitro toxicity, pharmacokinetics, and efficacy in a mouse peritonitis model of infection, which led to the discovery of compound 27. This new quinazolinone has potent activity against methicillin-resistant (MRSA) strains, low clearance, oral bioavailability and shows efficacy in a mouse neutropenic thigh infection model.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Centers for Disease Control (CDC). Antibiotic Resistance Threats in the United States, 2013; CDC: Atlanta, GA, 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

