Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury
- PMID: 27088797
- PMCID: PMC4855924
- DOI: 10.1172/JCI83000
Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury
Abstract
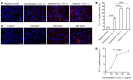
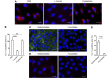
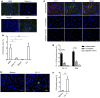
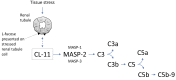
Physiochemical stress induces tissue injury as a result of the detection of abnormal molecular patterns by sensory molecules of the innate immune system. Here, we have described how the recently discovered C-type lectin collectin-11 (CL-11, also known as CL-K1 and encoded by COLEC11) recognizes an abnormal pattern of L-fucose on postischemic renal tubule cells and activates a destructive inflammatory response. We found that intrarenal expression of CL-11 rapidly increases in the postischemic period and colocalizes with complement deposited along the basolateral surface of the proximal renal tubule in association with L-fucose, the potential binding ligand for CL-11. Mice with either generalized or kidney-specific deficiency of CL-11 were strongly protected against loss of renal function and tubule injury due to reduced complement deposition. Ex vivo renal tubule cells showed a marked capacity for CL-11 binding that was induced by cell stress under hypoxic or hypothermic conditions and prevented by specific removal of L-fucose. Further analysis revealed that cell-bound CL-11 required the lectin complement pathway-associated protease MASP-2 to trigger complement deposition. Given these results, we conclude that lectin complement pathway activation triggered by ligand-CL-11 interaction in postischemic tissue is a potent source of acute kidney injury and is amenable to sugar-specific blockade.
Figures
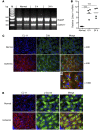
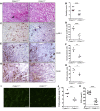
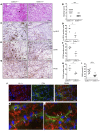

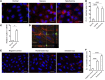
Comment in
-
Acute kidney injury: Collectin-11: a signal for complement activation.Nat Rev Nephrol. 2016 Jul;12(7):378. doi: 10.1038/nrneph.2016.68. Epub 2016 May 3. Nat Rev Nephrol. 2016. PMID: 27140851 No abstract available.
Similar articles
-
l-Fucose prevention of renal ischaemia/reperfusion injury in Mice.FASEB J. 2020 Jan;34(1):822-834. doi: 10.1096/fj.201901582R. Epub 2019 Nov 27. FASEB J. 2020. PMID: 31914693 Free PMC article.
-
Deconstructing the Lectin Pathway in the Pathogenesis of Experimental Inflammatory Arthritis: Essential Role of the Lectin Ficolin B and Mannose-Binding Protein-Associated Serine Protease 2.J Immunol. 2017 Sep 1;199(5):1835-1845. doi: 10.4049/jimmunol.1700119. Epub 2017 Jul 24. J Immunol. 2017. PMID: 28739878 Free PMC article.
-
Structure and function of collectin liver 1 (CL-L1) and collectin 11 (CL-11, CL-K1).Immunobiology. 2012 Sep;217(9):851-63. doi: 10.1016/j.imbio.2011.12.008. Epub 2012 Feb 4. Immunobiology. 2012. PMID: 22475410 Review.
-
Collectin Kidney 1 Plays an Important Role in Innate Immunity against Streptococcus pneumoniae Infection.J Innate Immun. 2017;9(2):217-228. doi: 10.1159/000453316. Epub 2017 Jan 10. J Innate Immun. 2017. PMID: 28068663 Free PMC article.
-
Fucose as a new therapeutic target in renal transplantation.Pediatr Nephrol. 2021 May;36(5):1065-1073. doi: 10.1007/s00467-020-04588-2. Epub 2020 May 29. Pediatr Nephrol. 2021. PMID: 32472330 Free PMC article. Review.
Cited by
-
Complement Inhibition in Kidney Transplantation: Where Are We Now?BioDrugs. 2023 Jan;37(1):5-19. doi: 10.1007/s40259-022-00567-1. Epub 2022 Dec 13. BioDrugs. 2023. PMID: 36512315 Free PMC article. Review.
-
Complement inhibitors in pediatric kidney diseases: new therapeutic opportunities.Pediatr Nephrol. 2024 May;39(5):1387-1404. doi: 10.1007/s00467-023-06120-8. Epub 2023 Sep 21. Pediatr Nephrol. 2024. PMID: 37733095 Review.
-
Role of the lectin pathway of complement in hematopoietic stem cell transplantation-associated endothelial injury and thrombotic microangiopathy.Exp Hematol Oncol. 2021 Dec 19;10(1):57. doi: 10.1186/s40164-021-00249-8. Exp Hematol Oncol. 2021. PMID: 34924021 Free PMC article. Review.
-
Delayed graft function post renal transplantation: a review on animal models and therapeutics.Am J Physiol Renal Physiol. 2023 Dec 1;325(6):F817-F825. doi: 10.1152/ajprenal.00146.2023. Epub 2023 Oct 19. Am J Physiol Renal Physiol. 2023. PMID: 37855040 Free PMC article. Review.
-
Pilot Study of Sodium-Glucose Cotransporter 2 Inhibitor Empagliflozin Shows Reduced Intrarenal Complement Activation in Patients With Diabetes and CKD.Kidney Int Rep. 2024 Nov 18;10(2):591-595. doi: 10.1016/j.ekir.2024.11.014. eCollection 2025 Feb. Kidney Int Rep. 2024. PMID: 39990876 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

