Safety of Eplerenone for Kidney-Transplant Recipients with Impaired Renal Function and Receiving Cyclosporine A
- PMID: 27088859
- PMCID: PMC4835088
- DOI: 10.1371/journal.pone.0153635
Safety of Eplerenone for Kidney-Transplant Recipients with Impaired Renal Function and Receiving Cyclosporine A
Abstract
Background: Animal studies have highlighted the role of vascular mineralocorticoid receptor during Cyclosporine A-induced nephrotoxicity. Mineralocorticoid receptor antagonists could improve kidney survival but are not commonly used during renal impairment and in association with several immunosuppressive drugs due to a supposed higher risk of adverse events. We tested the tolerance of eplerenone according to its expected adverse events: hyperkalemia, metabolic acidosis, hypotension, acute kidney failure, or any other adverse event.
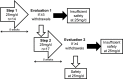
Methods: We conducted a single-center, prospective, open-label study in 31 kidney-transplant recipients with impaired renal function (30 and 50 mL/min/1.73 m2) and receiving cyclosporine A. All patients received eplerenone 25 mg/d for 8 weeks. Serum potassium, renal function and expected adverse events were closely monitored.
Results: Eight patients experienced mild hyperkalemia (>5 mmol/L), one moderate hyperkalemia (>5.5 mmol/L) and had to receive potassium-exchange resin. No severe hyperkalemia (>6 mmol/L) occurred. One acute kidney failure was observed, secondary to diarrhea. Basal serum potassium and bicarbonate were independently associated with a higher risk of developing mild hyperkalemia (>5 mmol/L) under treatment (OR 6.5, p = 0.003 and 0.7, p = 0.007, respectively). A cut-off value of 4.35 mmol/L for basal serum potassium was the best factor to predict the risk of developing mild hyperkalemia (>5 mmol/L).
Conclusions: Until eGFR falls to 30 mL/min/1.73 m2, eplerenone could be safely given to kidney-transplant recipients receiving cyclosporine A, if kalemia is closely monitored. When renal function is impaired and if basal kalemia is >4.35 mmol/L, then clinicians should properly balance risk and benefit of eplerenone use and offer dietary advice. An adequately powered prospective randomized study is now needed to test its efficiency (and safety) in this population.
Trial registration: ClinicalTrials.gov NCT01834768.
Conflict of interest statement
Figures


References
-
- English J, Evan A, Houghton DC, Bennett WM. Cyclosporine-induced acute renal dysfunction in the rat. Evidence of arteriolar vasoconstriction with preservation of tubular function. Transplantation. 1987;44(1):135–41. Epub 1987/07/01. . - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

