The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy
- PMID: 27088890
- PMCID: PMC4834814
- DOI: 10.1097/j.pain.0000000000000491
The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy
Abstract
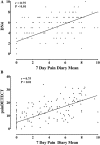
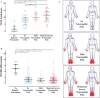
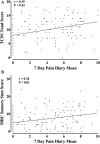
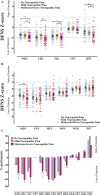
Disabling neuropathic pain (NeuP) is a common sequel of diabetic peripheral neuropathy (DPN). We aimed to characterise the sensory phenotype of patients with and without NeuP, assess screening tools for NeuP, and relate DPN severity to NeuP. The Pain in Neuropathy Study (PiNS) is an observational cross-sectional multicentre study. A total of 191 patients with DPN underwent neurological examination, quantitative sensory testing, nerve conduction studies, and skin biopsy for intraepidermal nerve fibre density assessment. A set of questionnaires assessed the presence of pain, pain intensity, pain distribution, and the psychological and functional impact of pain. Patients were divided according to the presence of DPN, and thereafter according to the presence and severity of NeuP. The DN4 questionnaire demonstrated excellent sensitivity (88%) and specificity (93%) in screening for NeuP. There was a positive correlation between greater neuropathy severity (r = 0.39, P < 0.01), higher HbA1c (r = 0.21, P < 0.01), and the presence (and severity) of NeuP. Diabetic peripheral neuropathy sensory phenotype is characterised by hyposensitivity to applied stimuli that was more marked in the moderate/severe NeuP group than in the mild NeuP or no NeuP groups. Brush-evoked allodynia was present in only those with NeuP (15%); the paradoxical heat sensation did not discriminate between those with (40%) and without (41.3%) NeuP. The "irritable nociceptor" subgroup could only be applied to a minority of patients (6.3%) with NeuP. This study provides a firm basis to rationalise further phenotyping of painful DPN, for instance, stratification of patients with DPN for analgesic drug trials.
Figures
Comment in
-
Somatosensory phenotyping for better translation in neuropathic pain?Pain. 2016 May;157(5):995-996. doi: 10.1097/j.pain.0000000000000505. Pain. 2016. PMID: 26835784 No abstract available.
References
-
- Baron R, Förster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol 2012;11:999–1005. - PubMed
-
- Bastien C, Vallières A, Morin C. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. - PubMed
-
- Bennett M. The LANSS Pain Scale: the leeds assessment of neuropathic symptoms and signs. PAIN 2001;92:147–57. - PubMed
-
- Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F, Lasischka F, Neuhuber WL, Kichko TI, Konrade I, Elvert R, Mier W, Pirags V, Lukic IK, Morcos M, Dehmer T, Rabbani N, Thornalley PJ, Edelstein D, Nau C, Forbes J, Humpert PM, Schwaninger M, Ziegler D, Stern DM, Cooper ME, Haberkorn U, Brownlee M, Reeh PW, Nawroth PP. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med 2012;18:926–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

