Daily Home Spirometry: An Effective Tool for Detecting Progression in Idiopathic Pulmonary Fibrosis
- PMID: 27089018
- PMCID: PMC5067818
- DOI: 10.1164/rccm.201511-2152OC
Daily Home Spirometry: An Effective Tool for Detecting Progression in Idiopathic Pulmonary Fibrosis
Abstract
Rationale: Recent clinical trial successes have created an urgent need for earlier and more sensitive endpoints of disease progression in idiopathic pulmonary fibrosis (IPF). Domiciliary spirometry permits more frequent measurement of FVC than does hospital-based assessment, which therefore affords the opportunity for a more granular insight into changes in IPF progression.
Objectives: To determine the feasibility and reliability of measuring daily FVC in individuals with IPF.
Methods: Subjects with IPF were given handheld spirometers and instruction on how to self-administer spirometry. Subjects recorded daily FEV1 and FVC for up to 490 days. Clinical assessment and hospital-based spirometry was undertaken at 6 and 12 months, and outcome data were collected for 3 years.
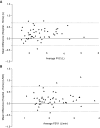
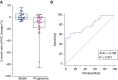
Measurements and main results: Daily spirometry was recorded by 50 subjects for a median period of 279 days (range, 13-490 d). There were 18 deaths during the active study period. Home spirometry showed excellent correlation with hospital-obtained readings. The rate of decline in FVC was highly predictive of outcome and subsequent mortality when measured at 3 months (hazard ratio [HR], 1.040; 95% confidence interval [CI], 1.021-1.062; P ≤ 0.001), 6 months (HR, 1.024; 95% CI, 1.014-1.033; P < 0.001), and 12 months (HR, 1.012; 95% CI, 1.007-1.016; P = 0.001).
Conclusions: Measurement of daily home spirometry in patients with IPF is highly clinically informative and is feasible to perform for most of these patients. The relationship between mortality and rate of change of FVC at 3 months suggests that daily FVC may be of value as a primary endpoint in short proof-of-concept IPF studies.
Keywords: biomarker; clinical trials; interstitial lung disease; personalized medicine.
Figures




Comment in
-
What Gets Measured Gets Managed: Daily Home Spirometry in Idiopathic Pulmonary Fibrosis.Am J Respir Crit Care Med. 2016 Oct 15;194(8):926-927. doi: 10.1164/rccm.201605-0906ED. Am J Respir Crit Care Med. 2016. PMID: 27739884 No abstract available.
-
Reply: Daily Home Spirometry: A New Milestone in the Field of Pulmonary Fibrosis.Am J Respir Crit Care Med. 2016 Oct 15;194(8):1034-1035. doi: 10.1164/rccm.201606-1238LE. Am J Respir Crit Care Med. 2016. PMID: 27739889 No abstract available.
-
Daily Home Spirometry: A New Milestone in the Field of Pulmonary Fibrosis.Am J Respir Crit Care Med. 2016 Oct 15;194(8):1033-1034. doi: 10.1164/rccm.201605-1090LE. Am J Respir Crit Care Med. 2016. PMID: 27739891 No abstract available.
References
-
- Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–839. - PubMed
-
- Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. - PubMed
-
- du Bois RM, Albera C, Bradford WZ, Costabel U, Leff JA, Noble PW, Sahn SA, Valeyre D, Weycker D, King TE., Jr 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2014;43:1421–1429. - PubMed
-
- du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Raghu G, Sahn SA, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:459–466. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

