Glutamine supplementation to prevent morbidity and mortality in preterm infants
- PMID: 27089158
- PMCID: PMC7055588
- DOI: 10.1002/14651858.CD001457.pub6
Glutamine supplementation to prevent morbidity and mortality in preterm infants
Abstract
Background: Glutamine is a conditionally essential amino acid. Endogenous biosynthesis may be insufficient for tissue needs in states of metabolic stress. Evidence exists that glutamine supplementation improves clinical outcomes in critically ill adults. It has been suggested that glutamine supplementation may also benefit preterm infants.
Objectives: To determine the effects of glutamine supplementation on mortality and morbidity in preterm infants.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group. This included searches of the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 12), MEDLINE, EMBASE and Maternity and Infant Care (to December 2015), conference proceedings and previous reviews.
Selection criteria: Randomised or quasi-randomised controlled trials that compared glutamine supplementation versus no glutamine supplementation in preterm infants at any time from birth to discharge from hospital.
Data collection and analysis: We extracted data using the standard methods of the Cochrane Neonatal Review Group, with separate evaluation of trial quality and data extraction by two review authors. We synthesised data using a fixed-effect model and reported typical relative risk, typical risk difference and weighted mean difference.
Main results: We identified 12 randomised controlled trials in which a total of 2877 preterm infants participated. Six trials assessed enteral glutamine supplementation and six trials assessed parenteral glutamine supplementation. The trials were generally of good methodological quality. Meta-analysis did not find an effect of glutamine supplementation on mortality (typical relative risk 0.97, 95% confidence interval 0.80 to 1.17; risk difference 0.00, 95% confidence interval -0.03 to 0.02) or major neonatal morbidities including the incidence of invasive infection or necrotising enterocolitis. Three trials that assessed neurodevelopmental outcomes in children aged 18 to 24 months and beyond did not find any effects.
Authors' conclusions: The available trial data do not provide evidence that glutamine supplementation confers important benefits for preterm infants.
Conflict of interest statement
None.
Figures
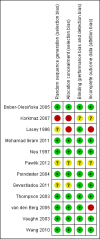













Update of
-
Glutamine supplementation to prevent morbidity and mortality in preterm infants.Cochrane Database Syst Rev. 2016 Jan 12;(1):CD001457. doi: 10.1002/14651858.CD001457.pub5. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2016 Apr 18;4:CD001457. doi: 10.1002/14651858.CD001457.pub6. PMID: 26755330 Updated.
References
References to studies included in this review
Bober‐Olesiñska 2005 {published data only}
-
- Bober‐Olesiñska K, Kornacka MK. Effects of glutamine supplemented parenteral nutrition on the incidence of necrotizing enterocolitis, nosocomial sepsis and length of hospital stay in very low birth weight infants. Medycyna Wieku Rozwojowego 2005;9:325‐33. - PubMed
Korkmaz 2007 {published data only}
-
- Korkmaz A, Yurdakök M, Yiğit S, Tekinalp G. Long‐term enteral glutamine supplementation in very low birth weight infants: effects on growth parameters. Turkish Journal of Pediatrics 2007;49:37‐44. [PUBMED: 17479642] - PubMed
Lacey 1996 {published data only}
-
- Lacey JM, Crouch JB, Benfell K, Ringer SA, Wilmore CK, Maguire D, Wilmore DW. The effects of glutamine‐supplemented parenteral nutrition in premature infants. Journal of Parenteral and Enteral Nutrition 1996;20:74‐80. [PUBMED: 8788268] - PubMed
Mohamad Ikram 2011 {published and unpublished data}
-
- Mohamad Ikram I, Quah BS, Noraida R, Djokomuljanto S, Faris Irfan CY, Rostenberghe H. A randomised controlled trial of glutamine‐enriched neonatal parenteral nutrition in Malaysia. Singapore Medical Journal 2011;52:356‐60. [PUBMED: 21633770] - PubMed
Neu 1997 {published data only}
-
- Dallas MJ, Bowling D, Roig JC, Auestad N, Neu J. Enteral glutamine supplementation for very‐low‐birth‐weight infants decreases hospital costs. Journal of Parenteral and Enteral Nutrition 1998;22:352‐6. [PUBMED: 9829607] - PubMed
-
- Neu J, Roig JC, Meetze WH, Veerman M, Carter C, Millsaps M, et al. Enteral glutamine supplementation for very low birth weight infants decreases morbidity. Journal of Pediatrics 1997;131:691‐9. [PMID: 9403648] - PubMed
-
- Roig JC, Meetze WH, Auestad N, Jasionowski T, Veerman M, McMurray CA, et al. Enteral glutamine supplementation for the very low birthweight infant: plasma amino acid concentrations. Journal of Nutrition 1996;126:1115S‐20S. [PUBMED: 8642443] - PubMed
Pawlik 2012 {published data only (unpublished sought but not used)}
-
- Pawlik D, Lauterbach R, Hurkala J, Radziszewska R. The effects of enteral administration of glutamine enriched solution in very low birth weight infants on reducing the symptoms of feeding intolerance. A prospective, randomised pilot study [Wplyw enteralnej podazy roztworu wzbogaconego glutamina, u noworodkow z bardzo mala urodzeniowa masa ciala, na organiczenie objawow nietolerancji karmienia. Prospektywne, randomizowane badanie pilotazowe]. Medycyna Wieku Rozwojowego 2012;XVI(3):206‐11. [PUBMED: 23378398] - PubMed
Poindexter 2004 {published data only}
-
- Poindexter B, Langer J, Dusick A, Ehrenkranz R, for the NICHD Neonatal Research Network. Early provision of parenteral amino acids in ELBW infants‐ relationship with growth and neurodevelopmental outcome at 18 months corrected age. Pediatric Research. 2004; Vol. 55:382A.
-
- Poindexter BB, Ehrenkrantz RA, Stoll BJ, Wright LL, Poole WK, Oh W, et al. Parenteral glutamine supplementation does not reduce the risk of morbidity or late‐onset sepsis in extremely‐low‐birthweight infants. Pediatrics 2004;113:1209‐15. [PMID: 15121931] - PubMed
-
- Poindexter BB, Ehrenkranz RA, Stoll BJ, Koch MA, Wright LL, Oh W, et al. Effect of parenteral glutamine supplementation on plasma amino acid concentrations in extremely low‐birth‐weight infants. American Journal of Clinical Nutrition 2003;77(3):737‐43. [PUBMED: 12600870] - PubMed
-
- Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. Journal of Pediatrics 2006;148(3):300‐5. [PUBMED: 16615955] - PubMed
Sevastiadou 2011 {published data only}
-
- Sevastiadou S, Malamitsi‐Puchner A, Costalos C, Skouroliakou M, Briana DD, Antsaklis A, et al. The impact of oral glutamine supplementation on the intestinal permeability and incidence of necrotizing enterocolitis/septicemia in premature neonates. Journal of Maternal‐Fetal Medicine 2011;24:1294‐300. [PUBMED: 21463215] - PubMed
Thompson 2003 {published data only}
-
- Thompson SW, McClure BG, Tubman TRJ. A randomised controlled trial of parenteral glutamine in ill very low birth weight neonates. Journal of Parenteral and Enteral Nutrition 2003;37:550‐3. [PUBMED: 14581795] - PubMed
van den Berg 2005 {published data only}
-
- Kieviet JF, Oosterlaan J, Vermeulen RJ, Pouwels PJW, Lafeber HN, Elburg RM. Effects of glutamine on brain development in very preterm children at school age. Pediatrics 2012;130(5):e1121‐7. [PUBMED: 23071202] - PubMed
-
- Kieviet JF, Oosterlaan J, Zwol A, Boehm G, Lafaber HN, Elburg RM. Effects of neonatal enteral glutamine supplementation on cognitive, motor, and behavioural outcomes in very preterm and/or very low birth weight children at school age. British Journal of Nutrition 2012;108(12):2215‐20. [PUBMED: 22313936] - PubMed
-
- Kieviet JF, Vujik PJ, Berg A, Lafaber HN, Oosterlaan J, Elburg RM. Glutamine effects on brain growth in very preterm children in the first year of life. Clinical Nutrition 2014;33(1):69‐74. [PUBMED: 23582234] - PubMed
-
- Zwol A, Berg A, Huisman J, Vermeulen RJ, Fetter WP, Twisk JW, et al. Neurodevelopmental outcomes of very low‐birth‐weight infants after enteral glutamine supplementation in the neonatal period. Acta Paediatrica 2008;97:562‐7. [PUBMED: 18394100] - PubMed
-
- Berg A, Fetter WP, Westerbeek EA, Vegt IM, Molen HR, Elburg RM. The effect of glutamine‐enriched enteral nutrition on intestinal permeability in very‐low‐birth‐weight infants: a randomized controlled trial. Journal of Parenteral and Enteral Nutrition 2006;30:408‐14. - PubMed
Vaughn 2003 {published data only}
-
- Vaughn P, Thomas P, Clark R, Neu J. Enteral glutamine supplementation and morbidity in low birth weight infants. Journal of Pediatrics 2003;142:662‐8. [PUBMED: 12838195] - PubMed
Wang 2010 {published data only}
-
- Wang Y, Cai W, Tao YX, Tang QY, Feng Y, Wu J. Clinical outcomes of glutamine supplementation in neonates. Chinese Journal of Clinical Nutrition 2009;17:259‐63.
-
- Wang Y, Cai W, Tao YX, Tang QY, Feng Y, Wu J. Glutamine supplementation in preterm infants receiving parenteral nutrition leads to an early improvement in liver function. Asia Pacific Journal of Clinical Nutrition 2013;22(4):530‐6. [PUBMED: 24231012] - PubMed
-
- Wang Y, Tao YX, Cai W, Tang QY, Feng Y, Wu J. Protective effect of parenteral glutamine supplementation on hepatic function in very low birth weight infants. Clinical Nutrition 2010;29:307‐11. [PUBMED: 20416995] - PubMed
References to studies excluded from this review
Barbosa 1999 {published data only}
-
- Barbosa E, Moreira AM, Goes JE, Faintuch J. Pilot study with a glutamine‐supplemented enteral formula in critically ill infants. Revista do Hospital dos Clinicas 1999;54(1):21‐4. [PUBMED: 10488597] - PubMed
Li 2007 {published data only}
-
- Li ZH, Wang DH, Dong M. Effect of parenteral glutamine supplementation in premature infants. Chinese Medical Journal 2007;120:140‐4. - PubMed
Strujis 2013 {published data only}
-
- Strujis MC, Schaible T, Elburg RM, Debauche C, Best H, Tibboel D. Efficacy and safety of a parenteral amino acid solution containing alanyl‐glutamine versus standard solution in infants: a first‐in‐man randomised double‐blind trial. Clinical Nutrition 2013;32(3):331‐7. [PUBMED: 23562219] - PubMed
References to ongoing studies
NCT00213668 {published data only}
-
- Effect of (enteral) Glutamine on Gastric Emptying and Length of Parenteral Nutrition in Premature Neonates. ClinicalTrials.gov 2010.
NCT01263041 {published data only}
-
- Effect of L‐arginine and Glutamine on Preterm (preterm). ClinicalTrials.gov 2011.
Additional references
Alverdy 1992
-
- Alverdy JA, Aoys E, Weiss‐Carrington P, Burke DA. The effect of glutamine‐enriched TPN on gut immune cellularity. Journal of Surgical Research 1992;52:34‐8. [PUBMED: 1548865] - PubMed
Avenell 2009
-
- Avenell A. Hot topics in parenteral nutrition. Current evidence and ongoing trials on the use of glutamine in critically‐ill patients and patients undergoing surgery. Proceedings of the Nutrition Society 2009;68:261‐8. [PUBMED: 19490739] - PubMed
Bergström 1974
-
- Bergström J, Fürst P, Norée LO, Vinnars E. Intracellular free amino acid concentrations in human muscle tissue. Journal of Applied Physiology 1974;36:693‐7. [PUBMED: 4829908] - PubMed
Brown 2014
Crowther 2009
-
- Crowther M, Avenell A, Culligan DJ. Systematic review and meta‐analyses of studies of glutamine supplementation in haematopoietic stem cell transplantation. Bone Marrow Transplantation 2009;44:413‐25. [PUBMED: 19270730] - PubMed
de Kieviet 2012
-
- Kieviet JF, Oosterlaan J, Zwol A, Boehm G, Lafaber HN, Elburg RM. Effects of neonatal enteral glutamine supplementation on cognitive, motor, and behavioural outcomes in very preterm and/or very low birth weight children at school age. British Journal of Nutrition 2012;108(12):2215‐20. [PUBMED: 22313936] - PubMed
GRADEpro 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro [Version 3.2 for Windows]. The GRADE Working Group, 2008.
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinshaw 1990
-
- Hinshaw DB, Burger JM. Protective effect of glutamine on endothelial cell ATP in oxidant injury. Journal of Surgical Research 1990;49:222‐7. - PubMed
Hopewell 2007a
Hopewell 2007b
Hopewell 2009
Klimberg 1990
-
- Klimberg VS, Salloum RM, Kasper M, Plumley DA, Dolson DJ, Hautamaki RD, et al. Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Archives of Surgery 1990;125:1040‐5. [PUBMED: 2378557] - PubMed
Krebs 1980
-
- Krebs H. Glutamine metabolism in the animal body. In: Mora J, Palacios R editor(s). Glutamine: metabolism, enzymology and regulation. New York: Academic Press, 1980:319‐29.
Lacey 1990
-
- Lacey J, Wilmore DW. Is glutamine a conditionally essential amino acid?. Nutrition Reviews 1990;48:297‐309. [PUBMED: 2080048] - PubMed
Lefebvre 2008
-
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomised trials published world‐wide ‐ the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library. Emerg Themes Epidemiol 2008;5:13. [PUBMED: 18826567] - PMC - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011.
Murray 2009
Newsholme 1985
-
- Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Quarterly Journal of Experimental Physiology 1985;70:473‐89. [PUBMED: 3909197] - PubMed
O'Dwyer 1989
-
- O'Dwyer ST, Smith RJ, Hwang TL, Wilmore DW. Maintenance of small bowel mucosa with glutamine enriched parenteral nutrition. Journal of Parenteral and Enteral Nutrition 1989;13:579‐85. [PUBMED: 2515303] - PubMed
Rombeau 1990
-
- Rombeau JL. A review of the effects of glutamine‐enriched diets on experimentally induced enterocolitis. Journal of Parenteral and Enteral Nutrition 1990;14:100S‐105S. [PUBMED: 2119454] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
van der Hulst 1993
-
- Hulst RRW, Kreel BK, Meyenfeldt MF, Brummer RJM, Arends JW, Deutz NEP, et al. Glutamine and the preservation of gut integrity. Lancet 1993;334:1363‐5. [PUBMED: 8098788] - PubMed
van Zwol 2011
-
- Zwol A, Neu J, Elburg RM. Long‐term effects of neonatal glutamine‐enriched nutrition in very‐low‐birth‐weight infants. Nutrition Reviews 2011;69:2‐8. [PUBMED: 21198630] - PubMed
Windmueller 1982
-
- Windmueller HG. Glutamine utilization by the small intestine. Advances in Enzymology and Related Areas of Molecular Biology 1982;53:201‐37. [PUBMED: 7036679] - PubMed
Wischmeyer 2008
-
- Wischmeyer PE. Glutamine: role in critical illness and ongoing clinical trials. Current Opinion in Gastroenterology 2008;24:190‐7. [PUBMED: 18301270] - PubMed
Young 2011
Ziegler 1990
-
- Ziegler TR, Benfell K, Smith RJ, Young LS, Brown E, Ferrari‐Baliviera E, et al. Safety and metabolic effects of L‐glutamine administration in humans. Journal of Parenteral and Enteral Nutrition 1990;14:137S‐146S. [PUBMED: 2119459] - PubMed
References to other published versions of this review
Moe‐Byrne 2012
Tubman 1999
Tubman 2001
-
- Tubman TRJ, Thompson SW. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001457.pub2] - DOI
Tubman 2005
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

