ROBUST: Lenalidomide-R-CHOP versus placebo-R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma
- PMID: 27089170
- PMCID: PMC5551933
- DOI: 10.2217/fon-2016-0130
ROBUST: Lenalidomide-R-CHOP versus placebo-R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma
Abstract
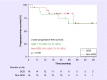
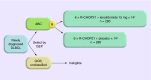
Activated B-cell-like (ABC) diffuse large B-cell lymphoma (DLBCL), the major constituent of nongerminal center B-cell-like (non-GCB) DLBCL, is associated with poorer survival outcomes than GCB-type DLBCL. In Phase II studies, lenalidomide combined with R-CHOP (R(2)-CHOP) improved outcomes relative to historical R-CHOP in newly diagnosed DLBCL, particularly in non-GCB cases. ROBUST (CC-5013-DLC-002) is a randomized, double-blind, global, Phase III study of oral lenalidomide (15 mg, days 1-14) plus R-CHOP21 × 6 versus placebo-R-CHOP21 × 6 in patients with previously untreated ABC-type DLBCL. Subtyping is done within 3 calendar days by central laboratory gene-expression profiling of formalin-fixed paraffin-embedded biopsy tissue. The primary end point is progression-free survival. Secondary end points include response rates, overall survival and health-related quality of life.
Keywords: chemotherapy; hematologic/lymphoma; molecular oncology.
Conflict of interest statement
Figures

References
-
- Bachy E, Salles G. Treatment approach to newly diagnosed diffuse large B-cell lymphoma. Semin. Hematol. 2015;52(2):107–118. - PubMed
-
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The non-Hodgkin's lymphoma classification project. Blood. 1997;89(11):3909–3918. - PubMed
-
- Harris NL, Jaffe ES, Stein H, et al. A revised European–American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361–1392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
