Linkers Having a Crucial Role in Antibody-Drug Conjugates
- PMID: 27089329
- PMCID: PMC4849017
- DOI: 10.3390/ijms17040561
Linkers Having a Crucial Role in Antibody-Drug Conjugates
Abstract
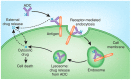
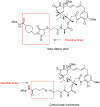
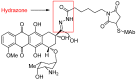
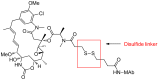
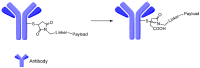
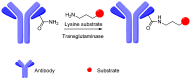
Antibody-drug conjugates (ADCs) comprised of a desirable monoclonal antibody, an active cytotoxic drug and an appropriate linker are considered to be an innovative therapeutic approach for targeted treatment of various types of tumors and cancers, enhancing the therapeutic parameter of the cytotoxic drug and reducing the possibility of systemic cytotoxicity. An appropriate linker between the antibody and the cytotoxic drug provides a specific bridge, and thus helps the antibody to selectively deliver the cytotoxic drug to tumor cells and accurately releases the cytotoxic drug at tumor sites. In addition to conjugation, the linkers maintain ADCs' stability during the preparation and storage stages of the ADCs and during the systemic circulation period. The design of linkers for ADCs is a challenge in terms of extracellular stability and intracellular release, and intracellular circumstances, such as the acid environment, the reducing environment and cathepsin, are considered as the catalysts to activate the triggers for initiating the cleavage of ADCs. This review discusses the linkers used in the clinical and marketing stages for ADCs and details the fracture modes of the linkers for the further development of ADCs.
Keywords: antibody–drug conjugates; attachment site; cytotoxic drug; linker; monoclonal antibody; tumor.
Figures












References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

