Frequency Dependence of Ultrasound Neurostimulation in the Mouse Brain
- PMID: 27090861
- PMCID: PMC4899295
- DOI: 10.1016/j.ultrasmedbio.2016.02.012
Frequency Dependence of Ultrasound Neurostimulation in the Mouse Brain
Abstract
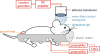
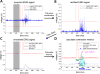
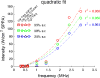
Ultrasound neuromodulation holds promise as a non-invasive technique for neuromodulation of the central nervous system. However, much remains to be determined about how the technique can be transformed into a useful technology, including the effect of ultrasound frequency. Previous studies have demonstrated neuromodulation in vivo using frequencies <1 MHz, with a trend toward improved efficacy with lower frequency. However, using higher frequencies could offer improved ultrasound spatial resolution. We investigate the ultrasound neuromodulation effects in mice at various frequencies both below and above 1 MHz. We find that frequencies up to 2.9 MHz can still be effective for generating motor responses, but we also confirm that as frequency increases, sonications require significantly more intensity to achieve equivalent efficacy. We argue that our results provide evidence that favors either a particle displacement or a cavitation-based mechanism for the phenomenon of ultrasound neuromodulation.
Keywords: Brain stimulation; Cavitation; Electromyography (EMG); Particle displacement; Radiation force; Ultrasound neuromodulation; Ultrasound neurostimulation.
Copyright © 2016 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Figures



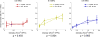




References
-
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol. 1991;17:179–185. - PubMed
-
- Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, Desalles A, Min BK, Yoo SS. A review of low-intensity focused ultrasound pulsation. Brain Stimul Elsevier Inc. 2011;4:125–136. - PubMed
-
- Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, Aubry J-F. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr Biol. 2013;23:2430–3. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

