Drugs for the acute treatment of migraine in children and adolescents
- PMID: 27091010
- PMCID: PMC6516975
- DOI: 10.1002/14651858.CD005220.pub2
Drugs for the acute treatment of migraine in children and adolescents
Abstract
Background: Numerous medications are available for the acute treatment of migraine in adults, and some have now been approved for use in children and adolescents in the ambulatory setting. A systematic review of acute treatment of migraine medication trials in children and adolescents will help clinicians make evidence-informed management choices.
Objectives: To assess the effects of pharmacological interventions by any route of administration versus placebo for migraine in children and adolescents 17 years of age or less. For the purposes of this review, children were defined as under 12 years of age and adolescents 12 to 17 years of age.
Search methods: We searched seven bibliographic databases and four clinical trial registers as well as gray literature for studies through February 2016.
Selection criteria: We included prospective randomized controlled clinical trials of children and adolescents with migraine, comparing acute symptom relieving migraine medications with placebo in the ambulatory setting.
Data collection and analysis: Two reviewers screened titles and abstracts and reviewed the full text of potentially eligible studies. Two independent reviewers extracted data for studies meeting inclusion criteria. We calculated the risk ratios (RRs) and number needed to treat for an additional beneficial outcome (NNTB) for dichotomous data. We calculated the risk difference (RD) and number needed to treat for an additional harmful outcome (NNTH) for proportions of adverse events. The percentage of pain-free patients at two hours was the primary efficacy outcome measure. We used adverse events to evaluate safety and tolerability. Secondary outcome measures included headache relief, use of rescue medication, headache recurrence, presence of nausea, and presence of vomiting. We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created 'Summary of findings' tables.
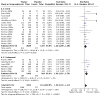
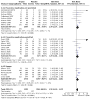
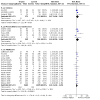
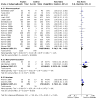
Main results: We identified a total of 27 randomized controlled trials (RCTs) of migraine symptom-relieving medications, in which 9158 children and adolescents were enrolled and 7630 (range of mean age between 8.2 and 14.7 years) received medication. Twenty-four studies focused on drugs in the triptan class, including almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan, sumatriptan + naproxen sodium, and zolmitriptan. Other medications studied included paracetamol (acetaminophen), ibuprofen, and dihydroergotamine (DHE). More than half of the studies evaluated sumatriptan. All but one study reported adverse event data. Most studies presented a low or unclear risk of bias, and the overall quality of evidence, according to GRADE criteria, was low to moderate, downgraded mostly due to imprecision and inconsistency. Ibuprofen was more effective than placebo for producing pain freedom at two hours in two small studies that included 162 children (RR 1.87, 95% confidence interval (CI) 1.15 to 3.04) with low quality evidence (due to imprecision). Paracetamol was not superior to placebo in one small study of 80 children. Triptans as a class of medication were superior to placebo in producing pain freedom in 3 studies involving 273 children (RR 1.67, 95% CI 1.06 to 2.62, NNTB 13) (moderate quality evidence) and 21 studies involving 7026 adolescents (RR 1.32, 95% CI 1.19 to 1.47, NNTB 6) (moderate quality evidence). There was no significant difference in the effect sizes between studies involving children versus adolescents. Triptans were associated with an increased risk of minor (non-serious) adverse events in adolescents (RD 0.13, 95% CI 0.08 to 0.18, NNTH 8), but studies did not report any serious adverse events. The risk of minor adverse events was not significant in children (RD 0.06, 95% CI - 0.04 to 0.17, NNTH 17). Sumatriptan plus naproxen sodium was superior to placebo in one study involving 490 adolescents (RR 3.25, 95% CI 1.78 to 5.94, NNTB 6) (moderate quality evidence). Oral dihydroergotamine was not superior to placebo in one small study involving 13 children.
Authors' conclusions: Low quality evidence from two small trials shows that ibuprofen appears to improve pain freedom for the acute treatment of children with migraine. We have only limited information on adverse events associated with ibuprofen in the trials included in this review. Triptans as a class are also effective at providing pain freedom in children and adolescents but are associated with higher rates of minor adverse events. Sumatriptan plus naproxen sodium is also effective in treating adolescents with migraine.
Conflict of interest statement
Lawrence Richer: no relevant conflicts of interest to declare.
Lori Billinghurst: no relevant conflicts of interest to declare.
Kelly Russell: no relevant conflicts of interest to declare.
Ben Vandermeer: no relevant conflicts of interest to declare.
Tamara Durec: no relevant conflicts of interest to declare.
Ellen Crumley: no relevant conflicts of interest to declare.
Lisa Hartling: no relevant conflicts of interest to declare.
Terry Klassen: no relevant conflicts of interest to declare.
Meghan Linsdell: no relevant conflicts of interest to declare.
Figures






























Update of
References
References to studies included in this review
Ahonen 2004 {published data only}
Ahonen 2006 {published data only}
Callenbach 2007 {published and unpublished data}SUM30042
-
- SUM30042. A multi-centre, randomised, double-blind, placebo-controlled, cross-over study to investigate the efficacy, safety and tolerability of sumatriptan nasal spray (10mg or 20mg) in the treatment of migraine in patients aged 12-17. http://www.gsk-clinicalstudyregister.com/study/SUM30042#rs (accessed 24 June 2015).
Derosier 2012 {published and unpublished data}
Evers 2006 {published data only}
Fujita 2014 {published and unpublished data}SUM111035
-
- SUM111035. A randomized, multicenter, placebo-controlled, parallel group study to evaluate the efficacy and safety of oral sumatriptan for the acute treatment of migraine in children and adolescents. http://www.gsk-clinicalstudyregister.com/study/111035#ps (accessed 24 June 2015).
Hämäläinen 1997a {published data only}
Hämäläinen 1997b {published data only}
-
- Hämäläinen ML, Hoppu K, Santavuori P. Sumatriptan for migraine attacks in children: a randomized placebo-controlled study. Do children with migraine respond to oral sumatriptan differently from adults? Neurology 1997;48(4):1100-3. [PMID: ] - PubMed
Hämäläinen 1997c {published data only}
Hämäläinen 2002 {published and unpublished data}SUM30009
-
- Hämäläinen M, Jones M, Loftus J, Saiers J. Sumatriptan nasal spray for migraine: a review of studies in patients aged 17 years and younger. International Journal of Clinical Practice 2002;56(9):704-9. [PMID: ] - PubMed
-
- SUM30009. A single centre, placebo-controlled, double blind, randomised cross-over, single attack study evaluating the efficacy and tolerability of sumatriptan nasal spray 10 mg for the acute treatment of migraine in children suffering from refractory migraine with/without aura. http://www.gsk-clinicalstudyregister.com/study/SUM30009#rs (accessed 24 June 2015).
Ho 2012 {published data only}
-
- Ho TW, Pearlman E, Lewis D, Hämäläinen M, Connor K, Michelson D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized, double-blind, placebo-controlled trial using a novel adaptive enrichment design. Headache 2012;32(10):750-65. [DOI: 10.1177/0333102412451358] - DOI - PubMed
Lewis 2002 {published data only}
Lewis 2007 {published data only}
Linder 2008 {published and unpublished data}
NCT01211145 {published and unpublished data}NCT01211145
-
- NCT01211145. Zomig - treatment of acute migraine headache in adolescents (TEENZ). clinicaltrials.gov/ct2/show/NCT01211145 (accessed 24 June 2015).
Rothner 1997 {published and unpublished data}S2WA3012
-
- Rothner A, Edwards K, Kerr L, DeBussey S, Asgharnejad M. Efficacy and safety of naratriptan tablets in adolescent migraine. Journal of Neurological Sciences 1997;150(Suppl 1):S106.
-
- S2WA3012. A randomized, double-blind, placebo-controlled, parallel study to evaluate the efficacy, safety and tolerability of oral naratriptan in an adolescent migraine population. http://www.gsk-clinicalstudyregister.com/study/S2WA3012#rs (accessed 24 June 2015).
Rothner 1999a {published and unpublished data}SUMB2003
-
- Rothner D, Asgharnejad M. Tolerability of sumatriptan tablets in the acute treatment of migraine in adolescent patients: a review of data from clinical trials. European Journal of Neurology 1999;6(Suppl 3):106.
-
- SUMB2003. A double-blind, randomised, placebo-controlled study to compare the efficacy and safety of oral sumatriptan (25mg, 50mg and 100mg) in the acute treatment of migraine in adolescents. http://www.gsk-clinicalstudyregister.com/study/SUMB2003#rs (accessed 24 June 2015).
Rothner 1999b {published and unpublished data}S2CT37
-
- Rothner D, Asgharnejad M. Tolerability of sumatriptan tablets in the acute treatment of migraine in adolescent patients: a review of data from clinical trials. European Journal of Neurology 1999;6(Suppl 3):106.
Rothner 1999c {published and unpublished data}S2CT40
-
- Rother D, Asgharnejad M. Tolerability of sumatriptan tablets in the acute treatment of migraine in adolescent patients: a review of data from clinical trials. European Journal of Neurology 1999;6(Suppl 3):106. [GSK ID: S2CT40]
Rothner 2006 {published data only}
Ueberall 1999 {published data only}
-
- Ueberall MA, Wenzel D. Intranasal sumatriptan for the acute treatment of migraine in children. Neurology 1999;52(7):1507-10. [PMID: ] - PubMed
Visser 2004a {published data only}
-
- Visser WH, Winner P, Strohmaier K, Klipfel M, Peng Y, McCarroll K, et al. Rizatriptan 5 mg for the acute treatment of migraine in adolescents: results from a double-blind, single-attack study and two open-label, multiple-attack studies. Headache 2004;44:891-9. [DOI: 10.1111/j.1526-4610.2004.04171.x] - DOI - PubMed
Winner 1997 {published and unpublished data}SUMA2002
-
- Winner P, Prensky A, Linder S, DeBussey S, Asgharnejad M. Adolescent migraine: efficacy and safety of sumatriptan tablets. Journal of the Neurological Sciences 1997;150(Suppl 1):S172. [DOI: 10.1016/S0022-510X(97)85694-8] - DOI
Winner 2000 {published and unpublished data}SUMA3005
-
- Winner P, Rothner AD, Saper J, Nett R, Asgharnejad M, Laurenza A, et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics 2000;106(5):989-97. [PMID: ] - PubMed
Winner 2002 {published data only}
Winner 2006 {published data only}
-
- SUM30045. A double-blind, placebo-controlled, parallel group study to evaluate two dose levels (5mg And 20mg) of sumatriptan nasal spray in the acute treatment of a single migraine attack in adolescent migraineurs (12-17 years of age). http://www.gsk-clinicalstudyregister.com/study/SUM30045#rs (accessed 24 June 2015).
-
- Winner P, Rothner D, Webster CJ, Ames MH, Kori SH. Randomized, double-blind, placebo- controlled study of sumatriptan nasal spray in adolescent migraineurs. Neurology 2004;62(7 Suppl 5):A182.
Winner 2007 {published data only}
-
- Pitman V. Efficacy, safety and tolerability of oral eletriptan (40 mg) for the treatment of migraine in adolescents (12-17 years). Headache 2000;40(5):424-5.
References to studies excluded from this review
Brousseau 2004 {published data only}
Cady 2011 {published data only}
Gertsch 2011 {published data only}
-
- Gertsch EA, Loharuka S, Wolter-Warmerdam KG, Tong S, Kedia S. Intravenous magnesium as abortive treatment for headaches in children. Annals of Neurology 2011;70(S15):S167. [DOI: 10.1002/ana.22563] - DOI
NCT00355394 {unpublished data only}
-
- NCT00355394. Treatment of acute migraine headache in children. clinicaltrials.gov/show/NCT00355394 (accessed 3 February 2016).
Soriani 2001 {published data only}
-
- Soriani S, Battistella PA, Naccarella C, Tozzi E, Fiumana E, Fanaro S. Nimesulide and acetaminophen for the treatment of juvenile migraine: a study for comparison of efficacy, safety, and tolerability. Headache Quarterly 2001;12:233-6.
SUM40090 {published data only}
-
- SUM40090. The use of oral sumatriptan on compassionate grounds for the acute treatment of migraine in adolescent patients (ages 12 to 17). http://www.gsk-clinicalstudyregister.com/study/SUM40090#rs (accessed 24 June 2015).
Trautmann 2010 {published data only}
Winner 2011 {published data only}
-
- Winner P. Sumatriptan formulated with RT technologyTM as early intervention for migraine in adolescents. Journal of Pediatric Neurology 2011;9(2):169-75. [DOI: 10.3233/JPN-2011-0471] - DOI
References to studies awaiting assessment
Winner 2015 {published and unpublished data}
-
- NCT01016678. Treximet Early Intervention Adolescent Migraine (TEAM). clinicaltrials.gov/ct2/show/NCT01016678 (accessed 24 June 2015).
Additional references
Bille 1997
Bonfert 2013
Curtin 2002
Damen 2005
Davies 2000
Egger 1997
Eiland 2010
Goadsby 2008
GRADEPro 2015 [Computer program]
-
- GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2015. Available from www.gradepro.org.
Harbord 2006
Higgins 2002
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: www.cochrane-handbook.org.
ICHD‐2
ICHD‐3 beta
IHS 1988
-
- International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias, and facial pain. Cephalalgia 1988;8(Suppl 7):1-96. - PubMed
Lathyris 2007
Levy 2008
Lewis 2004
-
- Lewis D, Ashwal S, Hershey A, Hirtz D, Yonker M, Silberstein S. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology 2004;63(12):2215-24. [DOI: 10.1212/01.WNL.0000147332.41993.90] - DOI - PubMed
Lipton 2002
-
- Lipton RB, Hamelsky SW, Dayno JM. What do patients with migraine want from acute migraine treatment? Headache 2002;42(Suppl 1):3-9. [PMID: ] - PubMed
Major 2003
Pierce 2010
Powers 2003
-
- Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in childhood migraines: clinical impact and comparison to other chronic illnesses. Pediatrics 2003;112(1 Pt 1):e1-5. [PMID: ] - PubMed
Powers 2004
RevMan 2014 [Computer program]
-
- Review Manager. Version 5.3. The Nordic Cochrane Centre, Copenhagen: Cochrane, 2014.
Silver 2008
Stata 14 [Computer program]
-
- Stata Statistical Software: Release 14. StataCorp. College Station, TX: StataCorp LP, 2015.
Stovner 2007
Sun 2013
Suthisisang 2010
Tfelt‐Hansen 2012
Toldo 2012
Vollono 2011
Winner 1995
-
- Winner P, Martinez W, Mate L, Bello L. Classification of pediatric migraine: proposed revisions to the IHS criteria. Headache 1995;35(7):407-10. - PubMed
References to other published versions of this review
Richer 2009
-
- Richer LP. Practice Variation in the Treatment of Children with Migraine in the Emergency Department [MSc Thesis]. Edmonton, AB: University of Alberta, 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

