Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation
- PMID: 27091175
- PMCID: PMC4835808
- DOI: 10.1038/srep24474
Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation
Abstract
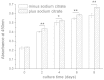
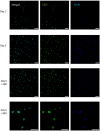
Alginate hydrogel is a popular biologically inert material that is widely used in 3D bioprinting, especially in extrusion-based printing. However, the printed cells in this hydrogel could not degrade the surrounding alginate gel matrix, causing them to remain in a poorly proliferating and non-differentiating state. Here, we report a novel study of the 3D printing of human corneal epithelial cells (HCECs)/collagen/gelatin/alginate hydrogel incubated with a medium containing sodium citrate to obtain degradation-controllable cell-laden tissue constructs. The 3D-printed hydrogel network with interconnected channels and a macroporous structure was stable and achieved high cell viability (over 90%). By altering the mole ratio of sodium citrate/sodium alginate, the degradation time of the bioprinting constructs can be controlled. Cell proliferation and specific marker protein expression results also revealed that with the help of sodium citrate degradation, the printed HCECs showed a higher proliferation rate and greater cytokeratin 3(CK3) expression, indicating that this newly developed method may help to improve the alginate bioink system for the application of 3D bioprinting in tissue engineering.
Figures






References
-
- Derby B. Printing and prototyping of tissues and scaffolds. Science. 338, 921–926 (2012). - PubMed
-
- Atala A. Tissue engineering and regenerative medicine: concepts for clinical application. Rejuv. Res. 7, 15–31 (2004). - PubMed
-
- Hollister S. J. Porous scaffold design for tissue engineering. Nat. mater. 4, 518–524 (2005). - PubMed
-
- Hutmacher D. W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 21, 2529–2543 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

