Ecological influences on the behaviour and fertility of malaria parasites
- PMID: 27091194
- PMCID: PMC4835847
- DOI: 10.1186/s12936-016-1271-0
Ecological influences on the behaviour and fertility of malaria parasites
Abstract
Background: Sexual reproduction in the mosquito is essential for the transmission of malaria parasites and a major target for transmission-blocking interventions. Male gametes need to locate and fertilize females in the challenging environment of the mosquito blood meal, but remarkably little is known about the ecology and behaviour of male gametes.
Methods: Here, a series of experiments explores how some aspects of the chemical and physical environment experienced during mating impacts upon the production, motility, and fertility of male gametes.
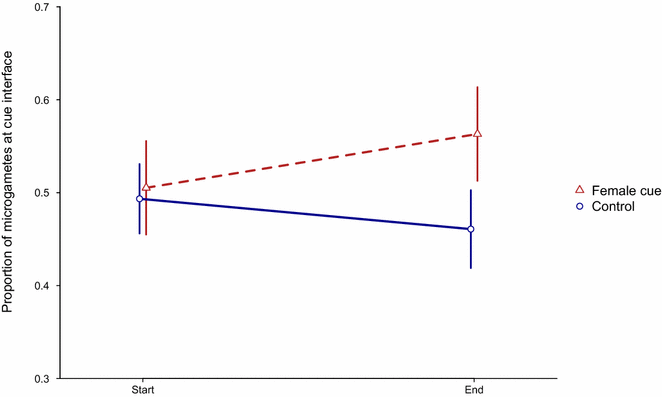
Results and conclusions: Specifically, the data confirm that: (a) rates of male gametogenesis vary when induced by the family of compounds (tryptophan metabolites) thought to trigger gamete differentiation in nature; and (b) complex relationships between gametogenesis and mating success exist across parasite species. In addition, the data reveal that (c) microparticles of the same size as red blood cells negatively affect mating success; and (d) instead of swimming in random directions, male gametes may be attracted by female gametes. Understanding the mating ecology of malaria parasites, may offer novel approaches for blocking transmission and explain adaptation to different species of mosquito vectors.
Keywords: Blood meal; Fertilization; Malaria; Microgamete; Transmission.
Figures
Similar articles
-
PfARID Regulates P. falciparum Malaria Parasite Male Gametogenesis and Female Fertility and Is Critical for Parasite Transmission to the Mosquito Vector.mBio. 2022 Jun 28;13(3):e0057822. doi: 10.1128/mbio.00578-22. Epub 2022 May 31. mBio. 2022. PMID: 35638735 Free PMC article.
-
Dynamic molecular events associated to Plasmodium berghei gametogenesis through proteomic approach.J Proteomics. 2018 May 30;180:88-98. doi: 10.1016/j.jprot.2017.11.009. Epub 2017 Nov 15. J Proteomics. 2018. PMID: 29155091
-
Time-of-day of blood-feeding: effects on mosquito life history and malaria transmission.Parasit Vectors. 2019 Jul 2;12(1):301. doi: 10.1186/s13071-019-3513-9. Parasit Vectors. 2019. PMID: 31262362 Free PMC article.
-
The microbiota, the malarial parasite, and the mosquito [MMM] - A three-sided relationship.Mol Biochem Parasitol. 2023 Feb;253:111543. doi: 10.1016/j.molbiopara.2023.111543. Epub 2023 Jan 13. Mol Biochem Parasitol. 2023. PMID: 36642385 Review.
-
The tripartite interactions between the mosquito, its microbiota and Plasmodium.Parasit Vectors. 2018 Mar 20;11(1):200. doi: 10.1186/s13071-018-2784-x. Parasit Vectors. 2018. PMID: 29558973 Free PMC article. Review.
Cited by
-
Transmission-Blocking Strategies Against Malaria Parasites During Their Mosquito Stages.Front Cell Infect Microbiol. 2022 Feb 16;12:820650. doi: 10.3389/fcimb.2022.820650. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35252033 Free PMC article. Review.
-
Nicotinamide and Demographic and Disease transitions: Moderation is Best.Int J Tryptophan Res. 2019 Jul 3;12:1178646919855940. doi: 10.1177/1178646919855940. eCollection 2019. Int J Tryptophan Res. 2019. PMID: 31320805 Free PMC article. Review.
-
The private life of malaria parasites: Strategies for sexual reproduction.Mol Biochem Parasitol. 2021 Jul;244:111375. doi: 10.1016/j.molbiopara.2021.111375. Epub 2021 May 20. Mol Biochem Parasitol. 2021. PMID: 34023299 Free PMC article. Review.
-
A computational lens for sexual-stage transmission, reproduction, fitness and kinetics in Plasmodium falciparum.Malar J. 2016 Sep 21;15(1):487. doi: 10.1186/s12936-016-1538-5. Malar J. 2016. PMID: 27653663 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

