Phase I Study of the Prolactin Receptor Antagonist LFA102 in Metastatic Breast and Castration-Resistant Prostate Cancer
- PMID: 27091421
- PMCID: PMC4861370
- DOI: 10.1634/theoncologist.2015-0502
Phase I Study of the Prolactin Receptor Antagonist LFA102 in Metastatic Breast and Castration-Resistant Prostate Cancer
Abstract
Lessons learned: Despite evidence for a role for prolactin signaling in breast and prostate tumorigenesis, a prolactin receptor-binding monoclonal antibody has not produced clinical efficacy.Increased serum prolactin levels may be a biomarker for prolactin receptor inhibition.Results from the pharmacokinetic and pharmacodynamics (PD) studies suggest that inappropriately long dosing intervals and insufficient exposure to LFA102 may have resulted in lack of antitumor efficacy.Based on preclinical data, combination therapy of LFA102 with those novel agents targeting hormonal pathways in metastatic castration-resistant prostate cancer and metastatic breast cancer is promising.Given the PD evidence of prolactin receptor blockade by LFA102, this drug has the potential to be used in conditions such as hyperprolactinemia that are associated with high prolactin levels.
Background: Prolactin receptor (PRLR) signaling is implicated in breast and prostate cancer. LFA102, a humanized monoclonal antibody (mAb) that binds to and inhibits the PRLR, has exhibited promising preclinical antitumor activity.
Methods: Patients with PRLR-positive metastatic breast cancer (MBC) or metastatic castration-resistant prostate cancer (mCRPC) received doses of LFA102 at 3-60 mg/kg intravenously once every 4 weeks. Objectives were to determine the maximum tolerated dose (MTD) and/or recommended dose for expansion (RDE) to investigate the safety/tolerability of LFA102 and to assess pharmacokinetics (PK), pharmacodynamics (PD), and antitumor activity.
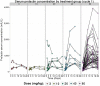
Results: A total of 73 patients were enrolled at 5 dose levels. The MTD was not reached because of lack of dose-limiting toxicities. The RDE was established at 60 mg/kg based on PK and PD analysis and safety data. The most common all-cause adverse events (AEs) were fatigue (44%) and nausea (33%) regardless of relationship. Grade 3/4 AEs reported to be related to LFA102 occurred in 4% of patients. LFA102 exposure increased approximately dose proportionally across the doses tested. Serum prolactin levels increased in response to LFA102 administration, suggesting its potential as a biomarker for PRLR inhibition. No antitumor activity was detected.
Conclusion: Treatment with LFA102 was safe and well tolerated, but did not show antitumor activity as monotherapy at the doses tested.
作者总结
经验
• 尽管有证据显示催乳素信号转导在乳腺和前列腺的肿瘤发生中发挥了一定作用, 但与催乳素受体结合的单克隆抗体尚未产生临床有效性。
• 血清催乳素水平升高可能作为催乳素受体抑制的生物标记物。
• 药代动力学和药效学 (PD) 研究提示, 不适当的长给药间歇及暴露剂量不足可能导致 LFA102 缺乏抗肿瘤有效性。
• 基于临床前数据, 采用 LFA102 与激素通路靶向新药联合方案用于治疗转移性去势抵抗性前列腺癌和转移性乳腺癌, 可望产生一定疗效。
鉴于催乳素受体受 LFA102 阻断的 PD 证据, 该药在诸如与高催乳素水平相关的高催乳素血症等情况下有一定应用潜力。
摘要
背景. 催乳素受体 (PRLR) 信号转导与乳腺和前列腺癌有关。人源化单克隆抗体 (mAb) LFA102 可与 PRLR 结合并抑制 PRLR, 其显示出的临床前抗肿瘤活性具有一定前景。
方法. 给予 PRLR 阳性的转移性乳腺癌 (MBC) 或转移性去势抵抗性前列腺癌 (mCRPC) 患者 LFA102 3∼60 mg/kg 静脉注射, 每 4 周一次。研究旨在确定最大耐受剂量 (MTD) 和 (或) 扩展阶段建议剂量 (RDE) 以调查 LFA102 的安全性/耐受性, 以及评估药代动力学 (PK) 、药效学 (PD) 和抗肿瘤活性。
结果. 共纳入 73 例患者, 接受了 5 个水平的给药剂量治疗。由于未发生剂量限制毒性, 因此未达到 MTD。依据 PK 和 PD 分析以及安全性数据, 确定 RDE 为 60 mg/kg。最常见的全因不良事件 (AE) 为乏力 (44%) 和恶心 (33%) , 无论是否与研究药物相关。 6%的患者发生了与 LFA102 相关的 3/4 级 AE。LA102 的暴露水平大致随检验剂量的增加而升高。血清催乳素水平随 LFA102 的给药而升高, 提示其具有作为 PRLR 抑制的生物标记物的潜力。研究未发现抗肿瘤活性。
结论. LFA102治疗安全且可耐受, 但在本研究检验剂量水平上单药使用未显示出抗肿瘤活性。The Oncologist 2016;21:535–536i
Trial registration: ClinicalTrials.gov NCT01338831.
©AlphaMed Press; the data published online to support this summary is the property of the authors.
Figures






Comment in
-
Targeting Prolactin Receptor (PRLR) Signaling in PRLR-Positive Breast and Prostate Cancer.Oncologist. 2016 May;21(5):523-6. doi: 10.1634/theoncologist.2016-0108. Epub 2016 Apr 22. Oncologist. 2016. PMID: 27107001 Free PMC article.
References
-
- Ben-Jonathan N, Liby K, McFarland M, et al. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol Metab. 2002;13:245–250. - PubMed
-
- Ormandy CJ, Camus A, Barra J, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. - PubMed
-
- Damiano JS, Wasserman E. Molecular pathways: Blockade of the PRLR signaling pathway as a novel antihormonal approach for the treatment of breast and prostate cancer. Clin Cancer Res. 2013;19:1644–1650. - PubMed
-
- Li H, Ahonen TJ, Alanen K, et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–4782. - PubMed
-
- Clevenger CV, Plank TL. Prolactin as an autocrine/paracrine factor in breast tissue. J Mammary Gland Biol Neoplasia. 1997;2:59–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

