A requirement for filopodia extension toward Slit during Robo-mediated axon repulsion
- PMID: 27091449
- PMCID: PMC5084274
- DOI: 10.1083/jcb.201509062
A requirement for filopodia extension toward Slit during Robo-mediated axon repulsion
Abstract
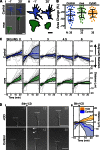
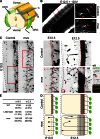
Axons navigate long distances through complex 3D environments to interconnect the nervous system during development. Although the precise spatiotemporal effects of most axon guidance cues remain poorly characterized, a prevailing model posits that attractive guidance cues stimulate actin polymerization in neuronal growth cones whereas repulsive cues induce actin disassembly. Contrary to this model, we find that the repulsive guidance cue Slit stimulates the formation and elongation of actin-based filopodia from mouse dorsal root ganglion growth cones. Surprisingly, filopodia form and elongate toward sources of Slit, a response that we find is required for subsequent axonal repulsion away from Slit. Mechanistically, Slit evokes changes in filopodium dynamics by increasing direct binding of its receptor, Robo, to members of the actin-regulatory Ena/VASP family. Perturbing filopodium dynamics pharmacologically or genetically disrupts Slit-mediated repulsion and produces severe axon guidance defects in vivo. Thus, Slit locally stimulates directional filopodial extension, a process that is required for subsequent axonal repulsion downstream of the Robo receptor.
© 2016 McConnell et al.
Figures





References
-
- Bear J.E., Svitkina T.M., Krause M., Schafer D.A., Loureiro J.J., Strasser G.A., Maly I.V., Chaga O.Y., Cooper J.A., Borisy G.G., and Gertler F.B.. 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 109:509–521. 10.1016/S0092-8674(02)00731-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

