Intrinsic network activity in tinnitus investigated using functional MRI
- PMID: 27091485
- PMCID: PMC4945432
- DOI: 10.1002/hbm.23204
Intrinsic network activity in tinnitus investigated using functional MRI
Abstract
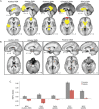
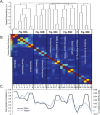
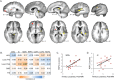
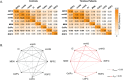
Tinnitus is an increasingly common disorder in which patients experience phantom auditory sensations, usually ringing or buzzing in the ear. Tinnitus pathophysiology has been repeatedly shown to involve both auditory and non-auditory brain structures, making network-level studies of tinnitus critical. In this magnetic resonance imaging (MRI) study, two resting-state functional connectivity (RSFC) approaches were used to better understand functional network disturbances in tinnitus. First, we demonstrated tinnitus-related reductions in RSFC between specific brain regions and resting-state networks (RSNs), defined by independent components analysis (ICA) and chosen for their overlap with structures known to be affected in tinnitus. Then, we restricted ICA to data from tinnitus patients, and identified one RSN not apparent in control data. This tinnitus RSN included auditory-sensory regions like inferior colliculus and medial Heschl's gyrus, as well as classically non-auditory regions like the mediodorsal nucleus of the thalamus, striatum, lateral prefrontal, and orbitofrontal cortex. Notably, patients' reported tinnitus loudness was positively correlated with RSFC between the mediodorsal nucleus and the tinnitus RSN, indicating that this network may underlie the auditory-sensory experience of tinnitus. These data support the idea that tinnitus involves network dysfunction, and further stress the importance of communication between auditory-sensory and fronto-striatal circuits in tinnitus pathophysiology. Hum Brain Mapp 37:2717-2735, 2016. © 2016 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: auditory; fMRI; functional connectivity; hearing loss; striatum; thalamus.
© 2016 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Figures
References
-
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD (2011): A baseline for the multivariate comparison of resting‐state networks. Front Syst Neurosci 5:2. - PMC - PubMed
-
- Behrens TEJ, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757. - PubMed
-
- Boyen K, Langers DRM, de Kleine E, van Dijk P (2013): Gray matter in the brain: Differences associated with tinnitus and hearing loss. Hear Res 295:67–76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

