Mortality risk and associated factors in HIV-exposed, uninfected children
- PMID: 27091659
- PMCID: PMC5021152
- DOI: 10.1111/tmi.12695
Mortality risk and associated factors in HIV-exposed, uninfected children
Abstract
Objective: With increasing maternal antiretroviral treatment (ART), the number of children newly infected with HIV has declined. However, the possible increased mortality in the large number of HIV-exposed, uninfected (HEU) children may be of concern. We quantified mortality risks among HEU children and reviewed associated factors.
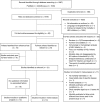
Methods: Systematic search of electronic databases (PubMed, Scopus). We included all studies reporting mortality of HEU children to age 60 months and associated factors. Relative risk of mortality between HEU and HIV-unexposed, uninfected (HUU) children was extracted where relevant. Inverse variance methods were used to adjust for study size. Random-effects models were fitted to obtain pooled estimates.
Results: A total of 14 studies were included in the meta-analysis and 13 in the review of associated factors. The pooled cumulative mortality in HEU children was 5.5% (95% CI: 4.0-7.2; I(2) = 94%) at 12 months (11 studies) and 11.0% (95% CI: 7.6-15.0; I(2) = 93%) at 24 months (four studies). The pooled risk ratios for the mortality in HEU children compared to HUU children in the same setting were 1.9 (95% CI: 0.9-3.8; I(2) = 93%) at 12 months (four studies) and 2.4 (95% CI: 1.1-5.1; I(2) = 93%) at 24 months (three studies).
Conclusion: Compared to HUU children, mortality risk in HEU children was about double at both age points, although the association was not statistically significant at 12 months. Interpretation of the pooled estimates is confounded by considerable heterogeneity between studies. Further research is needed to characterise the impact of maternal death and breastfeeding on the survival of HEU infants in the context of maternal ART, where current evidence is limited.
Objectif:
Avec l'augmentation du traitement antirétroviral (
Méthodes:
Recherche systématique dans les bases de données électroniques (PubMed, Scopus). Nous avons inclus toutes les études faisant état de la mortalité des enfants exposés, non infectés jusqu’à l’âge de 60 mois et les facteurs associés. Le risque relatif de mortalité entre les enfants exposés non inecftés et les enfants non exposés, non infectés par le
Résultats:
14 études ont été incluses dans la méta‐analyse et 13 dans l'analyse des facteurs associés. La mortalité poolée cumulative chez les enfants exposés non infectés était de 5,5% (
Conclusion:
Par rapport aux enfants non exposés, non infectés, le risque de mortalité chez les enfants exposés, non infectés était environ deux fois aux deux points d’âge, bien que l'association ne soit pas statistiquement significative à 12 mois. L'interprétation des estimations poolées est confondue par une hétérogénéité considérable entre les études. Des recherches supplémentaires sont nécessaires pour caractériser l'impact de la mortalité maternelle et l'allaitement maternel sur la survie des nourrissons exposés, non infectés, dans le cadre de l’
Objetivo:
Con el aumento de la terapia antiretroviral (
Métodos:
Búsqueda sistemática de bases de datos electrónicas (PubMed, Scopus). Hemos incluido todos los estudios que reportan mortalidad en niños
Resultados:
Se incluyeron 14 estudios dentro del meta‐análisis y 13 en la revisión de factores asociados. La mortalidad acumulada conjunta de niños
Conclusión:
Comparados con
Keywords: HIV; Mortalidad; Mortalité; VIH; child; enfant; facteur de risque; factores de riesgo; infant; lactantes; meta-analysis; meta-análisis; mortality; méta-analyse; niños; nourrisson; risk factor.
© 2016 The Authors. Tropical Medicine & International Health Published by John Wiley & Sons Ltd.
Figures


References
-
- UNAIDS . 2013 Report on the Global AIDS Epidemic. 2013.
-
- UNAIDS . How AIDS changed everything — MDG6: 15 years, 15 lessons of hope from the AIDS response. 2015.
-
- UNAIDS . Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2011. - PubMed
-
- Marinda E, Humphrey JH, Iliff PJ et al Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J 2007: 26: 519–526. - PubMed
-
- Brahmbhatt H, Kigozi G, Wabwire‐Mangen F et al Mortality in HIV‐infected and uninfected children of HIV‐infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr 2006: 41: 504–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

