Placental Growth Factor Reduces Blood Pressure in a Uteroplacental Ischemia Model of Preeclampsia in Nonhuman Primates
- PMID: 27091894
- PMCID: PMC4867111
- DOI: 10.1161/HYPERTENSIONAHA.116.07286
Placental Growth Factor Reduces Blood Pressure in a Uteroplacental Ischemia Model of Preeclampsia in Nonhuman Primates
Abstract
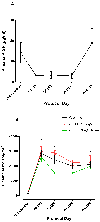
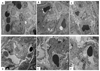
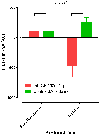
An imbalance in the angiogenesis axis during pregnancy manifests as clinical preeclampsia because of endothelial dysfunction. Circulating soluble fms-like tyrosine kinase 1 (sFLT-1) increases and placental growth factor (PlGF) reduces before and during disease. We investigated the clinical and biochemical effects of replenishing the reduced circulating PlGF with recombinant human PlGF (rhPlGF) and thus restoring the angiogenic balance. Hypertensive proteinuria was induced in a nonhuman primate (Papio hamadryas) by uterine artery ligation at 136 days gestation (of a 182-day pregnancy). Two weeks after uteroplacental ischemia, rhPlGF (rhPlGF, n=3) or normal saline (control, n=4) was administered by subcutaneous injection (100 μg/kg per day) for 5 days. Blood pressure was monitored by intra-arterial radiotelemetry and sFLT-1 and PlGF by ELISA. Uteroplacental ischemia resulted in experimental preeclampsia evidenced by increased blood pressure, proteinuria, and endotheliosis on renal biopsy and elevated sFLT-1. PlGF significantly reduced after uteroplacental ischemia. rhPlGF reduced systolic blood pressure in the treated group (-5.2±0.8 mm Hg; from 132.6±6.6 mm Hg to 124.1±7.6 mm Hg) compared with an increase in systolic blood pressure in controls (6.5±3 mm Hg; from 131.3±1.5 mm Hg to 138.6±1.5 mm Hg). Proteinuria reduced in the treated group (-72.7±55.7 mg/mmol) but increased in the control group. Circulating levels of total sFLT-1 were not affected by the administration of PlGF; however, a reduction in placental sFLT-1 mRNA expression was demonstrated. There was no significant difference between the weights or lengths of the neonates in the rhPlGF or control group; however, this study was not designed to assess fetal safety or outcomes. Increasing circulating PlGF by the administration of rhPlGF improves clinical parameters in a primate animal model of experimental preeclampsia.
Keywords: animal model; hypertension; placental growth factor; preeclampsia/pregnancy.
© 2016 American Heart Association, Inc.
Conflict of interest statement
Ravi Thadhani is co-inventor of patents related to diagnostics in the prediction of preeclampsia that have been out-licensed to diagnostic companies and has financial interest in Aggamin LLC. Ravi Thadhani also reports serving as a consultant to Roche diagnostics. S. Ananth Karumanchi is co-inventor of multiple patents related to the use of angiogenic proteins for the diagnosis and therapy of preeclampsia. These patents have been licensed to multiple companies. S. Ananth Karumanchi also reports serving as a consultant to Roche Diagnostics, Siemens and Thermofisher and has financial interest in Aggamin LLC.
Figures




Comment in
-
Placental Growth Factor: A Step Forward in the Search for a Treatment for Preeclampsia.Hypertension. 2016 Jun;67(6):1114-6. doi: 10.1161/HYPERTENSIONAHA.116.07378. Epub 2016 Apr 18. Hypertension. 2016. PMID: 27091897 No abstract available.
References
-
- Redman CW, Sargent IL, Staff AC. Ifpa senior award lecture: Making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta. 2014;35(Suppl):S20–25. - PubMed
-
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. - PubMed
-
- Savvidou MD, Akolekar R, Zaragoza E, Poon LC, Nicolaides KH. First trimester urinary placental growth factor and development of pre-eclampsia. Bjog. 2009;116:643–647. - PubMed
-
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. - PubMed
-
- Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

