Functional Analysis of the Role of Toxoplasma gondii Nucleoside Triphosphate Hydrolases I and II in Acute Mouse Virulence and Immune Suppression
- PMID: 27091930
- PMCID: PMC4936363
- DOI: 10.1128/IAI.00077-16
Functional Analysis of the Role of Toxoplasma gondii Nucleoside Triphosphate Hydrolases I and II in Acute Mouse Virulence and Immune Suppression
Abstract
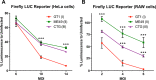
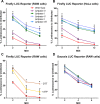
Bioluminescent reporter assays have been widely used to study the effect of Toxoplasma gondii on host gene expression. In the present study, we extend these studies by engineering novel reporter cell lines containing a gamma-activated sequence (GAS) element driving firefly luciferase (FLUC). In RAW264.7 macrophages, T. gondii type I strain (GT1) infection blocked interferon gamma (IFN-γ)-induced FLUC activity to a significantly greater extent than infection by type II (ME49) and type III (CTG) strains. Quantitative trait locus (QTL) analysis of progeny from a prior genetic cross identified a genomic region on chromosome XII that correlated with the observed strain-dependent phenotype. This QTL region contains two isoforms of the T. gondii enzyme nucleoside triphosphate hydrolase (NTPase) that were the prime candidates for mediating the observed strain-specific effect. Using reverse genetic analysis we show that deletion of NTPase I from a type I strain (RH) background restored the higher luciferase levels seen in the type II (ME49) strain. Rather than an effect on IFN-γ-dependent transcription, our data suggest that NTPase I was responsible for the strain-dependent difference in FLUC activity due to hydrolysis of ATP. We further show that NTPases I and II were not essential for tachyzoite growth in vitro or virulence in mice. Our study reveals that although T. gondii NTPases are not essential for immune evasion, they can affect ATP-dependent reporters. Importantly, this limitation was overcome using an ATP-independent Gaussia luciferase, which provides a more appropriate reporter for use with T. gondii infection studies.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Monoclonal antibodies against nucleoside triphosphate hydrolase-II can reduce the replication of Toxoplasma gondii.Parasitol Int. 2010 Jun;59(2):141-6. doi: 10.1016/j.parint.2009.12.007. Epub 2010 Jan 6. Parasitol Int. 2010. PMID: 20056166
-
The relationship between nucleoside triphosphate hydrolase (NTPase) isoform and Toxoplasma strain virulence in rat and human toxoplasmosis.Microbes Infect. 2003 Jul;5(9):797-806. doi: 10.1016/s1286-4579(03)00148-5. Microbes Infect. 2003. PMID: 12850206
-
Kinetics of the nucleoside triphosphate hydrolase of Toxoplasma gondii in mice with acute and chronic toxoplasmosis.Ann Trop Med Parasitol. 2002 Jan;96(1):35-41. doi: 10.1179/000349802125000493. Ann Trop Med Parasitol. 2002. PMID: 11989532
-
Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence.APMIS. 2009 May;117(5-6):458-76. doi: 10.1111/j.1600-0463.2009.02453.x. APMIS. 2009. PMID: 19400868 Free PMC article. Review.
-
On the determination of Toxoplasma gondii virulence in mice.Exp Parasitol. 2017 Mar;174:25-30. doi: 10.1016/j.exppara.2017.01.009. Epub 2017 Jan 30. Exp Parasitol. 2017. PMID: 28153801 Review.
Cited by
-
Toxoplasma gondii effector TgIST blocks type I interferon signaling to promote infection.Proc Natl Acad Sci U S A. 2019 Aug 27;116(35):17480-17491. doi: 10.1073/pnas.1904637116. Epub 2019 Aug 14. Proc Natl Acad Sci U S A. 2019. PMID: 31413201 Free PMC article.
-
Monoclonal Antibodies for Protozoan Infections: A Future Reality or a Utopic Idea?Front Med (Lausanne). 2021 Oct 12;8:745665. doi: 10.3389/fmed.2021.745665. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34712683 Free PMC article. Review.
-
The Expressed MicroRNA-mRNA Interactions of Toxoplasma gondii.Front Microbiol. 2018 Jan 4;8:2630. doi: 10.3389/fmicb.2017.02630. eCollection 2017. Front Microbiol. 2018. PMID: 29354114 Free PMC article.
-
Dissection of the in vitro developmental program of Hammondia hammondi reveals a link between stress sensitivity and life cycle flexibility in Toxoplasma gondii.Elife. 2018 May 22;7:e36491. doi: 10.7554/eLife.36491. Elife. 2018. PMID: 29785929 Free PMC article.
-
Monoclonal antibodies: From magic bullet to precision weapon.Mol Biomed. 2024 Oct 11;5(1):47. doi: 10.1186/s43556-024-00210-1. Mol Biomed. 2024. PMID: 39390211 Free PMC article. Review.
References
-
- Dubey JP. 2010. Toxoplasmosis of animals and humans. CRC Press, Boca Raton, FL.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

