Primary motor and sensory cortical areas communicate via spatiotemporally coordinated networks at multiple frequencies
- PMID: 27091982
- PMCID: PMC4983822
- DOI: 10.1073/pnas.1600788113
Primary motor and sensory cortical areas communicate via spatiotemporally coordinated networks at multiple frequencies
Abstract
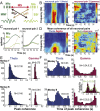
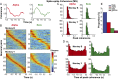
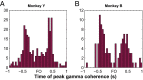
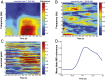
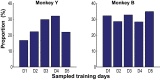
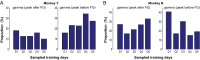
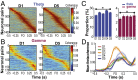
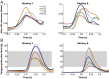
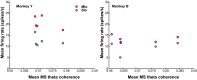
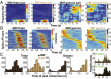
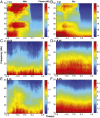
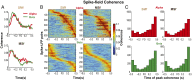
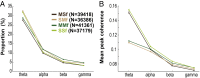
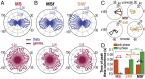
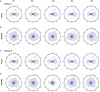
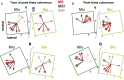
Skilled movements rely on sensory information to shape optimal motor responses, for which the sensory and motor cortical areas are critical. How these areas interact to mediate sensorimotor integration is largely unknown. Here, we measure intercortical coherence between the orofacial motor (MIo) and somatosensory (SIo) areas of cortex as monkeys learn to generate tongue-protrusive force. We report that coherence between MIo and SIo is reciprocal and that neuroplastic changes in coherence gradually emerge over a few days. These functional networks of coherent spiking and local field potentials exhibit frequency-specific spatiotemporal properties. During force generation, theta coherence (2-6 Hz) is prominent and exhibited by numerous paired signals; before or after force generation, coherence is evident in alpha (6-13 Hz), beta (15-30 Hz), and gamma (30-50 Hz) bands, but the functional networks are smaller and weaker. Unlike coherence in the higher frequency bands, the distribution of the phase at peak theta coherence is bimodal with peaks near 0° and ±180°, suggesting that communication between somatosensory and motor areas is coordinated temporally by the phase of theta coherence. Time-sensitive sensorimotor integration and plasticity may rely on coherence of local and large-scale functional networks for cortical processes to operate at multiple temporal and spatial scales.
Keywords: coherence; learning; motor cortex; orofacial cortex; somatosensory cortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
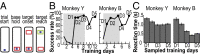




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

