Hysteresis in DNA compaction by Dps is described by an Ising model
- PMID: 27091987
- PMCID: PMC4983820
- DOI: 10.1073/pnas.1521241113
Hysteresis in DNA compaction by Dps is described by an Ising model
Abstract
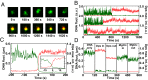
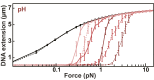
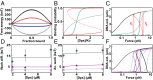
In all organisms, DNA molecules are tightly compacted into a dynamic 3D nucleoprotein complex. In bacteria, this compaction is governed by the family of nucleoid-associated proteins (NAPs). Under conditions of stress and starvation, an NAP called Dps (DNA-binding protein from starved cells) becomes highly up-regulated and can massively reorganize the bacterial chromosome. Although static structures of Dps-DNA complexes have been documented, little is known about the dynamics of their assembly. Here, we use fluorescence microscopy and magnetic-tweezers measurements to resolve the process of DNA compaction by Dps. Real-time in vitro studies demonstrated a highly cooperative process of Dps binding characterized by an abrupt collapse of the DNA extension, even under applied tension. Surprisingly, we also discovered a reproducible hysteresis in the process of compaction and decompaction of the Dps-DNA complex. This hysteresis is extremely stable over hour-long timescales despite the rapid binding and dissociation rates of Dps. A modified Ising model is successfully applied to fit these kinetic features. We find that long-lived hysteresis arises naturally as a consequence of protein cooperativity in large complexes and provides a useful mechanism for cells to adopt unique epigenetic states.
Keywords: DNA condensation; Dps; Ising model; cooperativity; hysteresis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Smith DE, Perkins TT, Chu S. Dynamical scaling of DNA diffusion coefficients. Macromolecules. 1996;29(4):1372–1373.
-
- Dillon SC, Dorman CJ. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol. 2010;8(3):185–195. - PubMed
-
- Dorman CJ. Function of nucleoid-associated proteins in chromosome structuring and transcriptional regulation. J Mol Microbiol Biotechnol. 2014;24(5-6):316–331. - PubMed
-
- Almirón M, Link AJ, Furlong D, Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992;6(12B):2646–2654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

