Structural characterization of the interaction of α-synuclein nascent chains with the ribosomal surface and trigger factor
- PMID: 27092002
- PMCID: PMC4983817
- DOI: 10.1073/pnas.1519124113
Structural characterization of the interaction of α-synuclein nascent chains with the ribosomal surface and trigger factor
Abstract
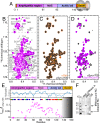
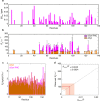
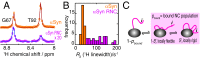
The ribosome is increasingly becoming recognized as a key hub for integrating quality control processes associated with protein biosynthesis and cotranslational folding (CTF). The molecular mechanisms by which these processes take place, however, remain largely unknown, in particular in the case of intrinsically disordered proteins (IDPs). To address this question, we studied at a residue-specific level the structure and dynamics of ribosome-nascent chain complexes (RNCs) of α-synuclein (αSyn), an IDP associated with Parkinson's disease (PD). Using solution-state nuclear magnetic resonance (NMR) spectroscopy and coarse-grained molecular dynamics (MD) simulations, we find that, although the nascent chain (NC) has a highly disordered conformation, its N-terminal region shows resonance broadening consistent with interactions involving specific regions of the ribosome surface. We also investigated the effects of the ribosome-associated molecular chaperone trigger factor (TF) on αSyn structure and dynamics using resonance broadening to define a footprint of the TF-RNC interactions. We have used these data to construct structural models that suggest specific ways by which emerging NCs can interact with the biosynthesis and quality control machinery.
Keywords: NMR spectroscopy; cotranslational folding; nascent chain; ribosome; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Bhushan S, et al. α-Helical nascent polypeptide chains visualized within distinct regions of the ribosomal exit tunnel. Nat Struct Mol Biol. 2010;17(3):313–317. - PubMed
-
- Lu J, Deutsch C. Secondary structure formation of a transmembrane segment in Kv channels. Biochemistry. 2005;44(23):8230–8243. - PubMed
-
- Zhang G, Ignatova Z. Folding at the birth of the nascent chain: Coordinating translation with co-translational folding. Curr Opin Struct Biol. 2011;21(1):25–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

