Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia
- PMID: 27092009
- PMCID: PMC4889352
- DOI: 10.1073/pnas.1517275113
Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia
Abstract
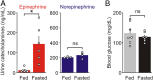
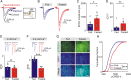
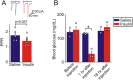
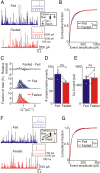
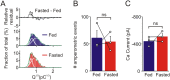
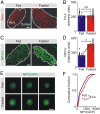
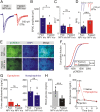
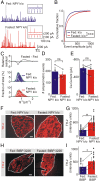
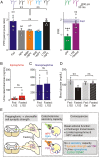
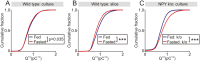
During fasting, activation of the counter-regulatory response (CRR) prevents hypoglycemia. A major effector arm is the autonomic nervous system that controls epinephrine release from adrenal chromaffin cells and, consequently, hepatic glucose production. However, whether modulation of autonomic function determines the relative strength of the CRR, and thus the ability to withstand food deprivation and maintain euglycemia, is not known. Here we show that fasting leads to altered transmission at the preganglionic → chromaffin cell synapse. The dominant effect is a presynaptic, long-lasting increase in synaptic strength. Using genetic and pharmacological approaches we show this plasticity requires neuropeptide Y, an adrenal cotransmitter and the activation of adrenal Y5 receptors. Loss of neuropeptide Y prevents a fasting-induced increase in epinephrine release and results in hypoglycemia in vivo. These findings connect plasticity within the sympathetic nervous system to a physiological output and indicate the strength of the final synapse in this descending pathway plays a decisive role in maintaining euglycemia.
Keywords: adrenal; autonomic nervous system; chromaffin cells; hypoglycemia; synaptic plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Food deprivation induces presynaptic plasticity in the autonomic nervous system.Proc Natl Acad Sci U S A. 2016 May 24;113(21):5766-7. doi: 10.1073/pnas.1605618113. Epub 2016 May 13. Proc Natl Acad Sci U S A. 2016. PMID: 27179009 Free PMC article. No abstract available.
Comment on
-
Food deprivation induces presynaptic plasticity in the autonomic nervous system.Proc Natl Acad Sci U S A. 2016 May 24;113(21):5766-7. doi: 10.1073/pnas.1605618113. Epub 2016 May 13. Proc Natl Acad Sci U S A. 2016. PMID: 27179009 Free PMC article. No abstract available.
Similar articles
-
Recurrent hypoglycemia inhibits the counterregulatory response by suppressing adrenal activity.J Clin Invest. 2018 Aug 31;128(9):3866-3871. doi: 10.1172/JCI91921. Epub 2018 Aug 6. J Clin Invest. 2018. PMID: 30080182 Free PMC article.
-
The β-Hydroxybutyrate-GPR109A Receptor Regulates Fasting-induced Plasticity in the Mouse Adrenal Medulla.Endocrinology. 2022 Jul 1;163(7):bqac077. doi: 10.1210/endocr/bqac077. Endocrinology. 2022. PMID: 35595517 Free PMC article.
-
Neuropeptide y gates a stress-induced, long-lasting plasticity in the sympathetic nervous system.J Neurosci. 2013 Jul 31;33(31):12705-17. doi: 10.1523/JNEUROSCI.3132-12.2013. J Neurosci. 2013. PMID: 23904607 Free PMC article.
-
Neuropeptides in the autonomic nervous system.CRC Crit Rev Clin Neurobiol. 1985;1(3):201-83. CRC Crit Rev Clin Neurobiol. 1985. PMID: 3915975 Review.
-
Neuropeptides in the sympathetic system: presence, plasticity, modulation, and implications.Ann Neurol. 1994 Jul;36(1):6-13. doi: 10.1002/ana.410360105. Ann Neurol. 1994. PMID: 8024263 Review.
Cited by
-
Intermittent Fasting: a Promising Approach for Preventing Vascular Dementia.J Lipid Atheroscler. 2019 May;8(1):1-7. doi: 10.12997/jla.2019.8.1.1. Epub 2019 Apr 5. J Lipid Atheroscler. 2019. PMID: 32821694 Free PMC article. Review.
-
Attenuation of the Counter-Regulatory Glucose Response in CVLM C1 Neurons: A Possible Explanation for Anorexia of Aging.Biomolecules. 2022 Mar 14;12(3):449. doi: 10.3390/biom12030449. Biomolecules. 2022. PMID: 35327640 Free PMC article.
-
Hypoglycemia-activated Hypothalamic Microglia Impairs Glucose Counterregulatory Responses.Sci Rep. 2019 Apr 17;9(1):6224. doi: 10.1038/s41598-019-42728-3. Sci Rep. 2019. PMID: 30996341 Free PMC article.
-
Molecular and functional diversity of the autonomic nervous system.Nat Rev Neurosci. 2025 Jul 3. doi: 10.1038/s41583-025-00941-2. Online ahead of print. Nat Rev Neurosci. 2025. PMID: 40610604 Review.
-
Does Ramadan Intermittent Fasting Affect the Fasting Blood Glucose Level among Type II Diabetic Patients?J Clin Med. 2023 Oct 18;12(20):6604. doi: 10.3390/jcm12206604. J Clin Med. 2023. PMID: 37892742 Free PMC article.
References
-
- Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369(4):362–372. - PubMed
-
- Cryer PE. Glucose counterregulation: Prevention and correction of hypoglycemia in humans. Am J Physiol. 1993;264(2 Pt 1):E149–E155. - PubMed
-
- Sivitz WI, et al. Effect of acute and antecedent hypoglycemia on sympathetic neural activity and catecholamine responsiveness in normal rats. Diabetes. 2001;50(5):1119–1125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

