Adiponectin Attenuates Angiotensin II-Induced Vascular Smooth Muscle Cell Remodeling through Nitric Oxide and the RhoA/ROCK Pathway
- PMID: 27092079
- PMCID: PMC4823273
- DOI: 10.3389/fphar.2016.00086
Adiponectin Attenuates Angiotensin II-Induced Vascular Smooth Muscle Cell Remodeling through Nitric Oxide and the RhoA/ROCK Pathway
Abstract
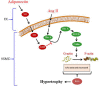
Introduction: Adiponectin (APN), an adipocytokine, exerts protective effects on cardiac remodeling, while angiotensin II (Ang II) induces hypertension and vascular remodeling. The potential protective role of APN on the vasculature during hypertension has not been fully elucidated yet. Here, we evaluate the molecular mechanisms of the protective role of APN in the physiological response of the vascular wall to Ang II.
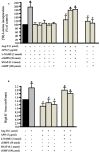
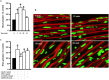
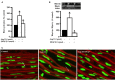
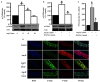
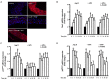
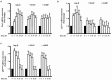
Methods and results: Rat aortic tissues were used to investigate the effect of APN on Ang II-induced vascular remodeling and hypertrophy. We investigated whether nitric oxide (NO), the RhoA/ROCK pathway, actin cytoskeleton remodeling, and reactive oxygen species (ROS) mediate the anti-hypertrophic effect of APN. Ang II-induced protein synthesis was attenuated by pre-treatment with APN, NO donor S-nitroso-N-acetylpenicillamine (SNAP), or cGMP. The hypertrophic response to Ang II was associated with a significant increase in RhoA activation and vascular force production, which were prevented by APN and SNAP. NO was also associated with inhibition of Ang II-induced phosphorylation of cofilin. In addition, immunohistochemistry revealed that 24 h Ang II treatment increased the F- to G-actin ratio, an effect that was inhibited by SNAP. Ang II-induced ROS formation and upregulation of p22(phox) mRNA expression were inhibited by APN and NO. Both compounds failed to inhibit Nox1 and p47(phox) expression.
Conclusion: Our results suggest that the anti-hypertrophic effects of APN are due, in part, to NO-dependent inhibition of the RhoA/ROCK pathway and ROS formation.
Keywords: VSMC; adiponectin; angiotensin II; nitric oxide; remodeling.
Figures
References
-
- Bagi Z., Feher A., Cassuto J., Akula K., Labinskyy N., Kaley G., et al. (2011). Increased availability of angiotensin AT 1 receptors leads to sustained arterial constriction to angiotensin II in diabetes – role for Rho-kinase activation. Br. J. Pharmacol. 163 1059–1068. 10.1111/j.1476-5381.2011.01307.x - DOI - PMC - PubMed
-
- Coles B., Fielding C. A., Rose-John S., Scheller J., Jones S. A., O’donnell V. B. (2007). Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am. J. Pathol. 171 315–325. 10.2353/ajpath.2007.061078 - DOI - PMC - PubMed
-
- Dai D. F., Johnson S. C., Villarin J. J., Chin M. T., Nieves-Cintron M., Chen T., et al. (2011). Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ. Res. 108 837–846. 10.1161/CIRCRESAHA.110.232306 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

