Cross-Regulation between N Metabolism and Nitric Oxide (NO) Signaling during Plant Immunity
- PMID: 27092169
- PMCID: PMC4824785
- DOI: 10.3389/fpls.2016.00472
Cross-Regulation between N Metabolism and Nitric Oxide (NO) Signaling during Plant Immunity
Abstract
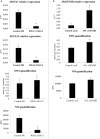
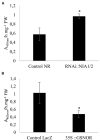
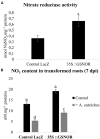
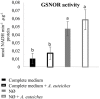
Plants are sessile organisms that have evolved a complex immune system which helps them cope with pathogen attacks. However, the capacity of a plant to mobilize different defense responses is strongly affected by its physiological status. Nitrogen (N) is a major nutrient that can play an important role in plant immunity by increasing or decreasing plant resistance to pathogens. Although no general rule can be drawn about the effect of N availability and quality on the fate of plant/pathogen interactions, plants' capacity to acquire, assimilate, allocate N, and maintain amino acid homeostasis appears to partly mediate the effects of N on plant defense. Nitric oxide (NO), one of the products of N metabolism, plays an important role in plant immunity signaling. NO is generated in part through Nitrate Reductase (NR), a key enzyme involved in nitrate assimilation, and its production depends on levels of nitrate/nitrite, NR substrate/product, as well as on L-arginine and polyamine levels. Cross-regulation between NO signaling and N supply/metabolism has been evidenced. NO production can be affected by N supply, and conversely NO appears to regulate nitrate transport and assimilation. Based on this knowledge, we hypothesized that N availability partly controls plant resistance to pathogens by controlling NO homeostasis. Using the Medicago truncatula/Aphanomyces euteiches pathosystem, we showed that NO homeostasis is important for resistance to this oomycete and that N availability impacts NO homeostasis by affecting S-nitrosothiol (SNO) levels and S-nitrosoglutathione reductase activity in roots. These results could therefore explain the increased resistance we noted in N-deprived as compared to N-replete M. truncatula seedlings. They open onto new perspectives for the studies of N/plant defense interactions.
Keywords: Aphanomyces euteiches; Medicago truncatula; nitric oxide homeostasis; nitrogen metabolism; plant immunity.
Figures


Similar articles
-
Nitrogen modulation of Medicago truncatula resistance to Aphanomyces euteiches depends on plant genotype.Mol Plant Pathol. 2018 Mar;19(3):664-676. doi: 10.1111/mpp.12550. Epub 2017 May 3. Mol Plant Pathol. 2018. PMID: 28296004 Free PMC article.
-
Aphanomyces euteiches cell wall fractions containing novel glucan-chitosaccharides induce defense genes and nuclear calcium oscillations in the plant host Medicago truncatula.PLoS One. 2013 Sep 23;8(9):e75039. doi: 10.1371/journal.pone.0075039. eCollection 2013. PLoS One. 2013. PMID: 24086432 Free PMC article.
-
Medicago TERPENE SYNTHASE 10 Is Involved in Defense Against an Oomycete Root Pathogen.Plant Physiol. 2019 Jul;180(3):1598-1613. doi: 10.1104/pp.19.00278. Epub 2019 Apr 23. Plant Physiol. 2019. PMID: 31015300 Free PMC article.
-
Nitrate Reductase Regulates Plant Nitric Oxide Homeostasis.Trends Plant Sci. 2017 Feb;22(2):163-174. doi: 10.1016/j.tplants.2016.12.001. Epub 2017 Jan 5. Trends Plant Sci. 2017. PMID: 28065651 Review.
-
[Research advance in nitrogen metabolism of plant and its environmental regulation].Ying Yong Sheng Tai Xue Bao. 2004 Mar;15(3):511-6. Ying Yong Sheng Tai Xue Bao. 2004. PMID: 15228008 Review. Chinese.
Cited by
-
Transcriptome Variations in Verticillium dahliae in Response to Two Different Inorganic Nitrogen Sources.Front Microbiol. 2021 Jul 28;12:712701. doi: 10.3389/fmicb.2021.712701. eCollection 2021. Front Microbiol. 2021. PMID: 34394062 Free PMC article.
-
The Dynamics of the Defense Strategy of Pea Induced by Exogenous Nitric Oxide in Response to Aphid Infestation.Int J Mol Sci. 2017 Feb 5;18(2):329. doi: 10.3390/ijms18020329. Int J Mol Sci. 2017. PMID: 28165429 Free PMC article.
-
Silicon and Nitrate Differentially Modulate the Symbiotic Performances of Healthy and Virus-Infected Bradyrhizobium-nodulated Cowpea (Vigna unguiculata), Yardlong Bean (V. unguiculata subsp. sesquipedalis) and Mung Bean (V. radiata).Plants (Basel). 2017 Sep 15;6(3):40. doi: 10.3390/plants6030040. Plants (Basel). 2017. PMID: 28914770 Free PMC article.
-
Metabolite profiling of susceptible and resistant wheat (Triticum aestivum) cultivars responding to Puccinia striiformis f. sp. tritici infection.BMC Plant Biol. 2023 Jun 1;23(1):293. doi: 10.1186/s12870-023-04313-9. BMC Plant Biol. 2023. PMID: 37264330 Free PMC article.
-
The Resistance of Oilseed Rape Microspore-Derived Embryos to Osmotic Stress Is Associated With the Accumulation of Energy Metabolism Proteins, Redox Homeostasis, Higher Abscisic Acid, and Cytokinin Contents.Front Plant Sci. 2021 Jun 11;12:628167. doi: 10.3389/fpls.2021.628167. eCollection 2021. Front Plant Sci. 2021. PMID: 34177973 Free PMC article.
References
-
- Abramowski D., Arasimowicz-Jelonek M., Izbiańska K., Billert H., Floryszak-Wieczorek J. (2015). Nitric oxide modulates redox-mediated defense in potato challenged with Phytophthora infestans. Eur. J. Plant Pathol. 143 237–260. 10.1007/s10658-015-0677-9 - DOI
-
- Astier J., Besson-Bard A., Lamotte O., Bertoldo J., Bourque S., Terenzi H., et al. (2012). Nitric oxide inhibits the ATPase activity of the chaperone-like AAA+ ATPase CDC48, a target for S-nitrosylation in cryptogein signalling in tobacco cells. Biochem. J. 447 249–260. 10.1042/BJ20120257 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

