Hepatotoxicity Induced by "the 3Ks": Kava, Kratom and Khat
- PMID: 27092496
- PMCID: PMC4849036
- DOI: 10.3390/ijms17040580
Hepatotoxicity Induced by "the 3Ks": Kava, Kratom and Khat
Abstract
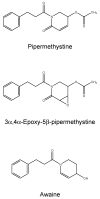
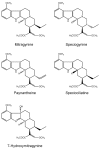
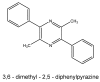
The 3Ks (kava, kratom and khat) are herbals that can potentially induce liver injuries. On the one hand, growing controversial data have been reported about the hepatotoxicity of kratom, while, on the other hand, even though kava and khat hepatotoxicity has been investigated, the hepatotoxic effects are still not clear. Chronic recreational use of kratom has been associated with rare instances of acute liver injury. Several studies and case reports have suggested that khat is hepatotoxic, leading to deranged liver enzymes and also histopathological evidence of acute hepatocellular degeneration. Numerous reports of severe hepatotoxicity potentially induced by kava have also been highlighted, both in the USA and Europe. The aim of this review is to focus on the different patterns and the mechanisms of hepatotoxicity induced by "the 3Ks", while trying to clarify the numerous aspects that still need to be addressed.
Keywords: hepatotoxicity; herb induced liver injury; herbals; kava; khat; kratom.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

