Activating and Tranquilizing Effects of First-Time Treatment with Aripiprazole, Olanzapine, Quetiapine, and Risperidone in Youth
- PMID: 27093218
- PMCID: PMC4931349
- DOI: 10.1089/cap.2015.0141
Activating and Tranquilizing Effects of First-Time Treatment with Aripiprazole, Olanzapine, Quetiapine, and Risperidone in Youth
Abstract
Objective: To assess activating and tranquilizing effects of second-generation antipsychotics (SGAs) in youth.
Methods: As part of the naturalistic inception cohort study, "Second-generation Antipsychotic Treatment Indication, Effectiveness and Tolerability in Youth (SATIETY)," subjective ratings of activating and tranquilizing symptoms were obtained monthly for 3 months from antipsychotic-naïve youth initiating SGAs using the Treatment Emergent Symptoms Scale (TESS). Discontinuation rates, and TESS-reported symptom rates, and severity were related to clinical and treatment parameters. Two compound measures of TESS were defined: presence of any daytime activating (ACTIVATION+) and sedating symptoms (SEDATION+).
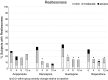
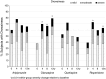
Results: In 327 antipsychotic-naïve youth originally initiating the four studied SGAs, discontinuation due to sedation was marginally highest with quetiapine (13.0%) followed by olanzapine (7.3%), risperidone (4.2%), and aripiprazole (2.0%) (p = 0.056). Two hundred fifty-seven antipsychotic-naïve youth (13.8 ± 3.6 years, male = 57.8%) initiated aripiprazole (n = 40), olanzapine (n = 45), quetiapine (n = 36), or risperidone (n = 135) and completed ≥1 postbaseline follow-up visit. Baseline prevalence of ACTIVATION+ (39.9%) or SEDATION+ (54.1%) did not differ between SGAs. Rates of both compound measures changed significantly over time (decrease for ACTIVATION+, p = 0.0002; increase for SEDATION+, p < 0.0001) with slight differences between SGAs, explained by lower rates of ACTIVATION+ with olanzapine (p = 0.002) and slightly higher rates of ACTIVATION+ with aripiprazole (p = 0.018) during follow-up, and lower rates of SEDATION+ with aripiprazole (p = 0.018). All four SGAs reduced insomnia (p = 0.001) and increased hypersomnia (p < 0.001). Postbaseline prevalence of drowsiness, the most frequent, but mild TESS complaint was 85%, without SGA differences. Younger age was associated with activating symptoms, higher age with sedating symptoms, and lower baseline functioning increased both. Psychomotor retardation rates were high in subjects with schizophrenia-spectrum disorders, whereas stimulant comedication was associated with psychomotor activation, regardless of diagnosis.
Conclusions: Although small SGA-specific differences in activating/sedating compound side effect measures were noted, independent predictors of single TESS ratings included clinical parameters, rather than specific SGAs, suggesting a need for carefully individualized treatment strategies.
Figures
References
-
- Addington DE, Pantelis C, Dineen M, Benattia I, Romano SJ: Efficacy and tolerability of ziprasidone versus risperidone in patients with acute exacerbation of schizophrenia or schizoaffective disorder: An 8-week, double-blind, multicenter trial. J Clin Psychiatry 65:1624–1633, 2004 - PubMed
-
- Aman MG, Gharabawi GM: Special Topic Advisory Panel on Transitioning to Risperidone Therapy in Patients with Mental Retardation and Developmental Disabilities: Treatment of behavior disorders in mental retardation: Report on transitioning to atypical antipsychotics, with an emphasis on risperidone. J Clin Psychiatry 65:1197–1210, 2004 - PubMed
-
- Azorin JM, Spiegel R, Remington G, Vanelle JM, Péré JJ, Giguere M, Bourdeix I: A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry 158:1305–1313, 2001 - PubMed
-
- Barzman DH, DelBello MP, Kowatch RA, Gernert B, Fleck DE, Pathak S, Rappaport K, Delgado SV, Campbell P, Strakowski SM: The effectiveness and tolerability of aripiprazole for pediatric bipolar disorders: A retrospective chart review. J Child Adolesc Psychopharmacol 14:593–600, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

