Optogenetic Stimulation of Arcuate Nucleus Kiss1 Neurons Reveals a Steroid-Dependent Glutamatergic Input to POMC and AgRP Neurons in Male Mice
- PMID: 27093227
- PMCID: PMC4884339
- DOI: 10.1210/me.2016-1026
Optogenetic Stimulation of Arcuate Nucleus Kiss1 Neurons Reveals a Steroid-Dependent Glutamatergic Input to POMC and AgRP Neurons in Male Mice
Abstract
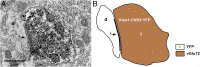
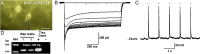
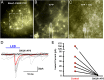
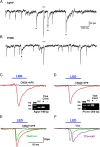
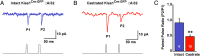
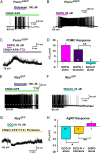
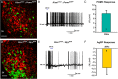
Kisspeptin (Kiss1) neurons are essential for reproduction, but their role in the control of energy balance and other homeostatic functions remains unclear. Proopiomelanocortin (POMC) and agouti-related peptide (AgRP) neurons, located in the arcuate nucleus (ARC) of the hypothalamus, integrate numerous excitatory and inhibitory inputs to ultimately regulate energy homeostasis. Given that POMC and AgRP neurons are contacted by Kiss1 neurons in the ARC (Kiss1(ARC)) and they express androgen receptors, Kiss1(ARC) neurons may mediate the orexigenic action of testosterone via POMC and/or AgRP neurons. Quantitative PCR analysis of pooled Kiss1(ARC) neurons revealed that mRNA levels for Kiss1 and vesicular glutamate transporter 2 were higher in castrated male mice compared with gonad-intact males. Single-cell RT-PCR analysis of yellow fluorescent protein (YFP) ARC neurons harvested from males injected with AAV1-EF1α-DIO-ChR2:YFP revealed that 100% and 88% expressed mRNAs for Kiss1 and vesicular glutamate transporter 2, respectively. Whole-cell, voltage-clamp recordings from nonfluorescent postsynaptic ARC neurons showed that low frequency photo-stimulation (0.5 Hz) of Kiss1-ChR2:YFP neurons elicited a fast glutamatergic inward current in POMC and AgRP neurons. Paired-pulse, photo-stimulation revealed paired-pulse depression, which is indicative of greater glutamate release, in the castrated male mice compared with gonad-intact male mice. Group I and group II metabotropic glutamate receptor agonists depolarized and hyperpolarized POMC and AgRP neurons, respectively, which was mimicked by high frequency photo-stimulation (20 Hz) of Kiss1(ARC) neurons. Therefore, POMC and AgRP neurons receive direct steroid- and frequency-dependent glutamatergic synaptic input from Kiss1(ARC) neurons in male mice, which may be a critical pathway for Kiss1 neurons to help coordinate energy homeostasis and reproduction.
Figures


References
-
- Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. - PubMed
-
- Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. - PubMed
-
- de Bond JA, Smith JT. Kisspeptin and energy balance in reproduction. Reproduction. 2014;147:R53–R63. - PubMed
-
- Roa J, Tena-Sempere M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. 2014;397:4–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

