Association between Polycystic Ovary Syndrome and Gut Microbiota
- PMID: 27093642
- PMCID: PMC4836746
- DOI: 10.1371/journal.pone.0153196
Association between Polycystic Ovary Syndrome and Gut Microbiota
Abstract
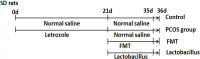
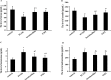
Polycystic ovary syndrome (PCOS) is the most frequent endocrinopathy in women of reproductive age. It is difficult to treat PCOS because of its complex etiology and pathogenesis. Here, we characterized the roles of gut microbiota on the pathogenesis and treatments in letrozole (a nonsteroidal aromatase inhibitor) induced PCOS rat model. Changes in estrous cycles, hormonal levels, ovarian morphology and gut microbiota by PCR-DGGE and real-time PCR were determined. The results showed that PCOS rats displayed abnormal estrous cycles with increasing androgen biosynthesis and exhibited multiple large cysts with diminished granulosa layers in ovarian tissues. Meanwhile, the composition of gut microbiota in letrozole-treated rats was different from that in the controls. Lactobacillus, Ruminococcus and Clostridium were lower while Prevotella was higher in PCOS rats when compared with control rats. After treating PCOS rats with Lactobacillus and fecal microbiota transplantation (FMT) from healthy rats, it was found that the estrous cycles were improved in all 8 rats in FMT group, and in 6 of the 8 rats in Lactobacillus transplantation group with decreasing androgen biosynthesis. Their ovarian morphologies normalized. The composition of gut microbiota restored in both FMT and Lactobacillus treated groups with increasing of Lactobacillus and Clostridium, and decreasing of Prevotella. These results indicated that dysbiosis of gut microbiota was associated with the pathogenesis of PCOS. Microbiota interventions through FMT and Lactobacillus transplantation were beneficial for the treatments of PCOS rats.
Conflict of interest statement
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

