Processing ribonucleotides incorporated during eukaryotic DNA replication
- PMID: 27093943
- PMCID: PMC5445644
- DOI: 10.1038/nrm.2016.37
Processing ribonucleotides incorporated during eukaryotic DNA replication
Abstract
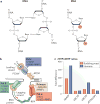
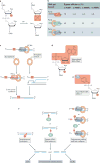
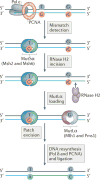
The information encoded in DNA is influenced by the presence of non-canonical nucleotides, the most frequent of which are ribonucleotides. In this Review, we discuss recent discoveries about ribonucleotide incorporation into DNA during replication by the three major eukaryotic replicases, DNA polymerases α, δ and ε. The presence of ribonucleotides in DNA causes short deletion mutations and may result in the generation of single- and double-strand DNA breaks, leading to genome instability. We describe how these ribonucleotides are removed from DNA through ribonucleotide excision repair and by topoisomerase I. We discuss the biological consequences and the physiological roles of ribonucleotides in DNA, and consider how deficiencies in their removal from DNA may be important in the aetiology of disease.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Martinez-Jimenez MI, et al. Alternative solutions and new scenarios for translesion DNA synthesis by human PrimPol. DNA Repair (Amst) 2015;29:127–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

