[The significance of serum IgD quantitation for evaluation of clinical efficacy in IgD multiple myeloma]
- PMID: 27093990
- PMCID: PMC7343088
- DOI: 10.3760/cma.j.issn.0253-2727.2016.04.008
[The significance of serum IgD quantitation for evaluation of clinical efficacy in IgD multiple myeloma]
Abstract
Objective: To investigate the significance of serum IgD quantitation in evaluation of clinical efficacy in IgD myeloma.
Methods: Serum IgD and free light chain (sFLC) levels were determined by immune scatter turbidimetry with SPA plus analysis machine in 29 patients with IgD multiple myeloma (MM) achieving VGPR or better response following previous treatments. The concurrent immunofixation electrophoresis (IFE) results were also incorporated and analyzed.
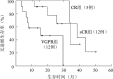
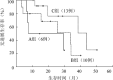
Results: Increased IgD levels were detected in 1 of 12 patients achieving sCR, 2 of 5 patients achieving CR and 4 of 12 patients achieving VGPR, respectively. The median progression-free survival (PFS) was 38.5 months, 34.1 months and 15.5 months for patients achieving sCR, CR and VGPR, respectively, with a significant difference between sCR and VGPR groups (P=0.022), and between CR and VGPR groups (P=0.018). There was no difference in overall survival (OS) among sCR, CR and VGPR groups (P>0.05). The median PFS were 7.8, 33.7 and 43.9 months, respectively for the patients with both abnormal sFLC ratios and IgD levels (6 cases, Group A), with either abnormal sFLC ratios or increased IgD levels (10 cases, Group B) or with normal sFLC ratios and IgD levels (13 cases, Group C). A significant PFS benefit of Group A over Group C was found (P=0.033), and no differences in terms of OS among three groups (P>0.05).
Conclusion: IgD levels may remain abnormal in IgD MM patients who have achieved VGPR or better response, and IgD quantitation represented a useful assay complementary to the current lab examinations. IgD quantitation assay was of significance in clinical efficacy evaluation and survival judgement, and should be incorporated into the evaluation parameters used for IgD MM in addition to sFLC and IFE assays.
目的: 评价血清IgD定量检测在IgD型多发性骨髓瘤(MM)患者疗效评估中的意义。
方法: 纳入29例临床疗效达到严格意义完全缓解(sCR)、完全缓解(CR)和非常好的部分缓解(VGPR)的IgD型MM患者,采用全自动SPAplus特定蛋白分析仪(散射免疫比浊法)进行IgD定量及血清游离轻链(sFLC)检测,结合同期血清免疫固定电泳(IFE)M蛋白检测结果评价IgD定量检测在疗效评估中的意义。
结果: ①sCR组(12例)、CR组(5例)、VGPR组(12例)分别有1、2、4例患者IgD定量结果异常升高。②全部29例患者中位随访24.7(8.6~41.5)个月,sCR组、CR组、VGPR组的中位无进展生存(PFS)期分别为38.5、34.1、15.5个月,sCR组与VGPR组差异有统计学意义(P=0.022),CR组与VGPR组差异有统计学意义(P=0.018),sCR组与CR组差异无统计学意义(P=0.846);sCR组、CR组、VGPR组的总生存(OS)期分别为41.5、37.7、19.1个月,差异无统计学意义(P>0.05)。③6例患者sFLC比值及IgD定量均异常升高(A组),10例患者sFLC比值异常或IgD定量异常升高(B组),13例患者sFLC比值及IgD定量正常(C组)。A组PFS期短于C组(7.8个月对43.9个月,P=0.033),B组与A、C组差异无统计学意义(33.7个月对7.8个月,P=0.404;33.7个月对43.9个月,P=0.121)。A、B、C组的OS期分别为11.8、37.5、47.1个月,差异无统计学意义(P>0.05)。
结论: 达到VGPR及以上疗效的IgD型MM患者仍存在IgD水平异常;联合应用IgD定量与sFLC及IFE检测可预测VGPR及以上疗效IgD型MM患者的PFS。
Figures
Similar articles
-
Prognostic Value of Serum Free Light Chains Measurements in Multiple Myeloma Patients.PLoS One. 2016 Nov 28;11(11):e0166841. doi: 10.1371/journal.pone.0166841. eCollection 2016. PLoS One. 2016. PMID: 27893836 Free PMC article.
-
[The roles of serum free light chain ratio in the diagnosis and prognosis of newly diagnosed multiple myeloma].Zhonghua Xue Ye Xue Za Zhi. 2016 May 14;37(5):377-82. doi: 10.3760/cma.j.issn.0253-2727.2016.05.005. Zhonghua Xue Ye Xue Za Zhi. 2016. PMID: 27210871 Free PMC article. Chinese.
-
[Detection of serum free light chain and its clinical significance in nonsecretory multiple myeloma].Zhonghua Xue Ye Xue Za Zhi. 2008 Feb;29(2):113-6. Zhonghua Xue Ye Xue Za Zhi. 2008. PMID: 18681313 Chinese.
-
Interpretation Difficulties of Serum Immunofixation Test in Immunoglobulin D Multiple Myeloma with Hidden Lambda Light Chains.Clin Lab. 2018 Jun 1;64(6):1065-1069. doi: 10.7754/Clin.Lab.2018.180109. Clin Lab. 2018. PMID: 29945318 Review.
-
Unusual myelomas: a review of IgD and IgE variants.Oncology (Williston Park). 2013 Aug;27(8):798-803. Oncology (Williston Park). 2013. PMID: 24133829 Review.
Cited by
-
Presentation Patterns, Diagnostic Markers, Management Strategies, and Outcomes of IgD Multiple Myeloma: A Systematic Review of Literature.Cureus. 2019 Feb 4;11(2):e4011. doi: 10.7759/cureus.4011. Cureus. 2019. PMID: 31001465 Free PMC article. Review.
-
Clinical characteristics and survival of patients with IgD multiple myeloma.Blood Sci. 2021 Apr 27;3(2):57-58. doi: 10.1097/BS9.0000000000000066. eCollection 2021 Apr. Blood Sci. 2021. PMID: 35402831 Free PMC article. No abstract available.
References
-
- Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants[J] Oncology (Williston Park) 2013;27(8):798–803. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous