Identification of a residue crucial for the angiostatic activity of human mini tryptophanyl-tRNA synthetase by focusing on its molecular evolution
- PMID: 27094087
- PMCID: PMC4837363
- DOI: 10.1038/srep24750
Identification of a residue crucial for the angiostatic activity of human mini tryptophanyl-tRNA synthetase by focusing on its molecular evolution
Abstract
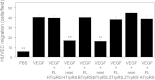
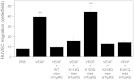
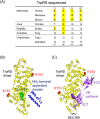
Human tryptophanyl-tRNA synthetase (TrpRS) exists in two forms: a full-length TrpRS and a mini TrpRS. We previously found that human mini, but not full-length, TrpRS is an angiostatic factor. Moreover, it was shown that the interaction between mini TrpRS and the extracellular domain of vascular endothelial (VE)-cadherin is crucial for its angiostatic activity. However, the molecular mechanism of the angiostatic activity of human mini TrpRS is only partly understood. In the present study, we investigated the effects of truncated (mini) form of TrpRS proteins from human, bovine, or zebrafish on vascular endothelial growth factor (VEGF)-stimulated chemotaxis of human umbilical vein endothelial cells (HUVECs). We show that both human and bovine mini TrpRSs inhibited VEGF-induced endothelial migration, whereas zebrafish mini TrpRS did not. Next, to identify residues crucial for the angiostatic activity of human mini TrpRS, we prepared several site-directed mutants based on amino acid sequence alignments among TrpRSs from various species and demonstrated that a human mini K153Q TrpRS mutant cannot inhibit VEGF-stimulated HUVEC migration and cannot bind to the extracellular domain of VE-cadherin. Taken together, we conclude that the Lys153 residue of human mini TrpRS is a VE-cadherin binding site and is therefore crucial for its angiostatic activity.
Figures




Similar articles
-
Oxidative stress-responsive intracellular regulation specific for the angiostatic form of human tryptophanyl-tRNA synthetase.Biochemistry. 2005 Jan 11;44(1):225-32. doi: 10.1021/bi048313k. Biochemistry. 2005. PMID: 15628863
-
An exposed cysteine residue of human angiostatic mini tryptophanyl-tRNA synthetase.Biochemistry. 2010 Apr 13;49(14):3156-60. doi: 10.1021/bi1000239. Biochemistry. 2010. PMID: 20225827
-
A short peptide insertion crucial for angiostatic activity of human tryptophanyl-tRNA synthetase.Nat Struct Mol Biol. 2004 Feb;11(2):149-56. doi: 10.1038/nsmb722. Epub 2004 Jan 11. Nat Struct Mol Biol. 2004. PMID: 14730354
-
Non-canonical functions of human cytoplasmic tyrosyl-, tryptophanyl- and other aminoacyl-tRNA synthetases.Enzymes. 2020;48:207-242. doi: 10.1016/bs.enz.2020.04.001. Epub 2020 Jun 12. Enzymes. 2020. PMID: 33837705 Review.
-
The IFN-γ/miniTrpRS signaling axis: An insight into the pathophysiology of osteoporosis and therapeutic potential.Cytokine Growth Factor Rev. 2022 Apr;64:7-11. doi: 10.1016/j.cytogfr.2022.01.005. Epub 2022 Jan 21. Cytokine Growth Factor Rev. 2022. PMID: 35115234 Review.
Cited by
-
The high-affinity tryptophan uptake transport system in human cells.Biochem Soc Trans. 2024 Jun 26;52(3):1149-1158. doi: 10.1042/BST20230742. Biochem Soc Trans. 2024. PMID: 38813870 Free PMC article. Review.
-
Unique roles of tryptophanyl-tRNA synthetase in immune control and its therapeutic implications.Exp Mol Med. 2019 Jan 7;51(1):1-10. doi: 10.1038/s12276-018-0196-9. Exp Mol Med. 2019. PMID: 30613102 Free PMC article. Review.
-
Tryptophanyl-tRNA synthetase mediates high-affinity tryptophan uptake into human cells.J Biol Chem. 2018 Jun 1;293(22):8428-8438. doi: 10.1074/jbc.RA117.001247. Epub 2018 Apr 17. J Biol Chem. 2018. PMID: 29666190 Free PMC article.
-
Tryptophan-Starved Human Cells Overexpressing Tryptophanyl-tRNA Synthetase Enhance High-Affinity Tryptophan Uptake via Enzymatic Production of Tryptophanyl-AMP.Int J Mol Sci. 2023 Oct 22;24(20):15453. doi: 10.3390/ijms242015453. Int J Mol Sci. 2023. PMID: 37895133 Free PMC article.
-
New insights into the cellular temporal response to proteostatic stress.Elife. 2018 Oct 12;7:e39054. doi: 10.7554/eLife.39054. Elife. 2018. PMID: 30272558 Free PMC article.
References
-
- Schimmel P. Aminoacyl-tRNA synthetases: General scheme of structure-functional relationships in the polypeptides and recognition of transfer RNAs. Annu. Rev. Biochem. 56, 125–158 (1987). - PubMed
-
- Wakasugi K. & Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284, 147–151 (1999). - PubMed
-
- Wakasugi K. & Schimmel P. Highly differentiated motifs responsible for two cytokine activities of a split human tRNA synthetase. J. Biol. Chem. 274, 23155–23159 (1999). - PubMed
-
- Wakasugi K. et al. Induction of angiogenesis by a fragment of human tyrosyl-tRNA synthetase. J Biol. Chem. 277, 20124–20126 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

