Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model
- PMID: 27094330
- PMCID: PMC4959521
- DOI: 10.1128/mBio.00417-16
Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model
Abstract
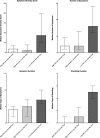
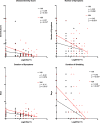
Despite long-term investment, influenza continues to be a significant worldwide problem. The cornerstone of protection remains vaccination, and approved vaccines seek to elicit a hemagglutination inhibition (HAI) titer of ≥1:40 as the primary correlate of protection. However, recent poor vaccine performance raises questions regarding the protection afforded and whether other correlates of protection should be targeted. A healthy volunteer challenge study was performed with a wild-type 2009 A(H1N1)pdm influenza A challenge virus at the NIH Clinical Center to evaluate two groups of participants with HAI titers of ≥1:40 and <1:40. The primary objective was to determine whether participants with HAI titers of ≥1:40 were less likely to develop mild to moderate influenza disease (MMID) after intranasal inoculation. HAI titers of ≥1:40 were protective against MMID but did not reduce the incidence of symptoms alone. Although the baseline HAI titer correlated with some reduction in disease severity measures, overall, the baseline NAI titer correlated more significantly with all disease severity metrics and had a stronger independent effect on outcome. This study demonstrates the importance of examining other immunological correlates of protection rather than solely HAI titers. This challenge study confirms the importance of NAI titer as a correlate and for the first time establishes that it can be an independent predictor of reduction of all aspects of influenza disease. This suggests that NAI titer may play a more significant role than previously thought and that neuraminidase immunity should be considered when studying susceptibility after vaccination and as a critical target in future influenza vaccine platforms.
Importance: This study represents the first time the current gold standard for evaluating influenza vaccines as set by the U.S. Food and Drug Administration and the European Medicines Agency Committee for Medicinal Products for Human Use, a "protective" hemagglutination inhibition (HAI) titer of ≥1:40, has been evaluated in a well-controlled healthy volunteer challenge study since the cutoff was established. We used our established wild-type influenza A healthy volunteer human challenge model to evaluate how well this antibody titer predicts a reduction in influenza virus-induced disease. We demonstrate that although higher HAI titer is predictive of some protection, there is stronger evidence to suggest that neuraminidase inhibition (NAI) titer is more predictive of protection and reduced disease. This is the first time NAI titer has been clearly identified in a controlled trial of this type to be an independent predictor of a reduction in all aspects of influenza.
Copyright © 2016 Memoli et al.
Figures
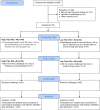
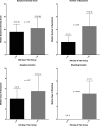
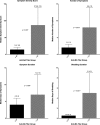
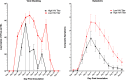

References
-
- Centers for Disease Control and Prevention 2010. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep 59:1057–1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
