The Eng1 β-Glucanase Enhances Histoplasma Virulence by Reducing β-Glucan Exposure
- PMID: 27094334
- PMCID: PMC4850272
- DOI: 10.1128/mBio.01388-15
The Eng1 β-Glucanase Enhances Histoplasma Virulence by Reducing β-Glucan Exposure
Abstract
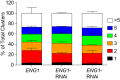
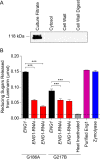
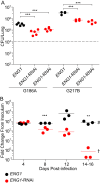
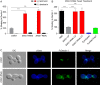
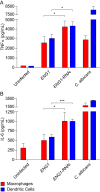
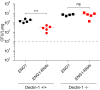
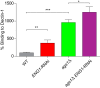
The fungal pathogen Histoplasma capsulatum parasitizes host phagocytes. To avoid antimicrobial immune responses, Histoplasma yeasts must minimize their detection by host receptors while simultaneously interacting with the phagocyte. Pathogenic Histoplasma yeast cells, but not avirulent mycelial cells, secrete the Eng1 protein, which is a member of the glycosylhydrolase 81 (GH81) family. We show that Histoplasma Eng1 is a glucanase that hydrolyzes β-(1,3)-glycosyl linkages but is not required for Histoplasma growth in vitro or for cell separation. However, Histoplasma yeasts lacking Eng1 function have attenuated virulence in vivo, particularly during the cell-mediated immunity stage. Histoplasma yeasts deficient for Eng1 show increased exposure of cell wall β-glucans, which results in enhanced binding to the Dectin-1 β-glucan receptor. Consistent with this, Eng1-deficient yeasts trigger increased tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) cytokine production from macrophages and dendritic cells. While not responsible for large-scale cell wall structure and function, the secreted Eng1 reduces levels of exposed β-glucans at the yeast cell wall, thereby diminishing potential recognition by Dectin-1 and proinflammatory cytokine production by phagocytes. In α-glucan-producing Histoplasma strains, Eng1 acts in concert with α-glucan to minimize β-glucan exposure: α-glucan provides a masking function by covering the β-glucan-rich cell wall, while Eng1 removes any remaining exposed β-glucans. Thus, Histoplasma Eng1 has evolved a specialized pathogenesis function to remove exposed β-glucans, thereby enhancing the ability of yeasts to escape detection by host phagocytes.
Importance: The success of Histoplasma capsulatum as an intracellular pathogen results, in part, from an ability to minimize its detection by receptors on phagocytic cells of the immune system. In this study, we showed that Histoplasma pathogenic yeast cells, but not avirulent mycelia, secrete a β-glucanase, Eng1, which reduces recognition of fungal cell wall β-glucans. We demonstrated that the Eng1 β-glucanase promotes Histoplasma virulence by reducing levels of surface-exposed β-glucans on yeast cells, thereby enabling Histoplasma yeasts to escape detection by the host β-glucan receptor, Dectin-1. As a consequence, phagocyte recognition of Histoplasma yeasts is reduced, leading to less proinflammatory cytokine production by phagocytes and less control of Histoplasma infection in vivo Thus, Histoplasma yeasts express two mechanisms to avoid phagocyte detection: masking of cell wall β-glucans by α-glucan and enzymatic removal of exposed β-glucans by the Eng1 β-glucanase.
Copyright © 2016 Garfoot et al.
Figures
Comment in
-
Trimming Surface Sugars Protects Histoplasma from Immune Attack.mBio. 2016 Apr 26;7(2):e00553-16. doi: 10.1128/mBio.00553-16. mBio. 2016. PMID: 27118584 Free PMC article.
References
-
- Wu-Hsieh B, Howard DH. 1984. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol 132:2593–2597. - PubMed
-
- Newman SL, Bucher C, Rhodes J, Bullock WE. 1990. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J Clin Invest 85:223–230. doi:10.1172/JCI114416. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
