Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure
- PMID: 27094549
- PMCID: PMC4956243
- DOI: 10.1177/1744806916641950
Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure
Abstract
Background: Opioids have become the mainstay for treatment of moderate to severe pain and are commonly used to treat surgical pain. While opioid administration has been shown to cause opioid-induced hyperalgesia and tolerance, interactions between opioid administration and surgery with respect to these problematic adaptations have scarcely been addressed. Accumulating evidence suggests opioids and nociceptive signaling may converge on epigenetic mechanisms in spinal cord to enhance or prolong neuroplastic changes. Epigenetic regulation of Bdnf (brain-derived neurotrophic factor) and Pdyn (prodynorphin) genes may be involved.
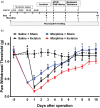
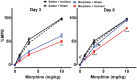
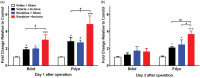
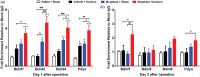
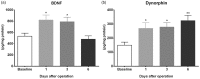
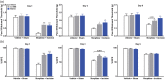
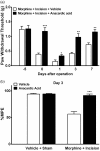
Results: Four days of ascending doses of morphine treatment caused opioid-induced hyperalgesia and reduced opioid analgesic efficacy in mice. Both opioid-induced hyperalgesia and the reduced opioid analgesic efficacy were enhanced in mice that received hindpaw incisions. The expression of Bdnf and Pdyn (qPCR) was increased after morphine treatment and incision. Chromatin immunoprecipitation assays demonstrated that the Pdyn and Bdnf promoters were more strongly associated with acetylated H3K9 after morphine plus incision than in the morphine or incision alone groups. Selective tropomyosin-related kinase B (ANA-12) and κ-opioid receptor (nor-binaltorphimine) antagonists were administered intrathecally, both reduced hyperalgesia one or three days after surgery. Administration of ANA-12 or nor-binaltorphimine attenuated the decreased morphine analgesic efficacy on day 1, but only nor-binaltorphimine was effective on day 3 after incision in opioid-exposed group. Coadministration of histone acetyltransferase inhibitor anacardic acid daily with morphine blocked the development of opioid-induced hyperalgesia and attenuated incision-enhanced hyperalgesia in morphine-treated mice. Anacardic acid had similar effects on analgesic tolerance, showing the involvement of histone acetylation in the interactions detected.
Conclusions: Spinal epigenetic changes involving Bdnf and Pdyn may contribute to the enhanced postoperative nociceptive sensitization and analgesic tolerance observed after continuous opioid exposure. Treatments blocking the epigenetically mediated up-regulation of these genes or administration of TrkB or κ-opioid receptor antagonists may improve the clinical utility of opioids, particularly after surgery.
Keywords: BDNF and dynorphin; Epigenetics; histone acetylation; incision; opioid-induced hyperalgesia.
© The Author(s) 2016.
Figures
References
-
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104: 570–587. - PubMed
-
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008; 24: 479–496. - PubMed
-
- Liang DY, Clark JD. Modulation of the NO/CO-cGMP signaling cascade during chronic morphine exposure in mice. Neurosci Lett 2004; 365: 73–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

