Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD
- PMID: 27094610
- PMCID: PMC4891748
- DOI: 10.2215/CJN.06890615
Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD
Abstract
Background and objectives: Roxadustat (FG-4592), an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that stimulates erythropoiesis, regulates iron metabolism, and reduces hepcidin, was evaluated in this phase 2b study for safety, efficacy, optimal dose, and dose frequency in patients with nondialysis CKD.
Design, setting, participants, & measurements: The 145 patients with nondialysis CKD and hemoglobin ≤10.5 g/dl were randomized into one of six cohorts of approximately 24 patients each with varying roxadustat starting doses (tiered weight and fixed amounts) and frequencies (two and three times weekly) followed by hemoglobin maintenance with roxadustat one to three times weekly. Treatment duration was 16 or 24 weeks. Intravenous iron was prohibited. The primary end point was the proportion of patients achieving hemoglobin increase of ≥1.0 g/dl from baseline and hemoglobin of ≥11.0 g/dl by week 17 (16 weeks of treatment). Secondary analyses included mean hemoglobin change from baseline, iron utilization, and serum lipids. Safety was evaluated by frequency/severity of adverse events.
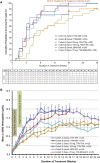
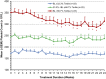
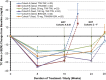
Results: Of the 145 patients enrolled, 143 were evaluable for efficacy. Overall, 92% of patients achieved hemoglobin response. Higher compared with lower starting doses led to earlier achievement of hemoglobin response. Roxadustat-induced hemoglobin increases were independent of baseline C-reactive protein levels and iron repletion status. Overall, over the first 16 treatment weeks, hepcidin levels decreased by 16.9% (P=0.004), reticulocyte hemoglobin content was maintained, and hemoglobin increased by a mean (±SD) of 1.83 (±0.09) g/dl (P<0.001). Overall mean total cholesterol level was reduced by a mean (±SD) of 26 (±30) mg/dl (P<0.001) after 8 weeks of therapy, independent of the use of statins or other lipid-lowering agents. No drug-related serious adverse events were reported.
Conclusions: In patients with nondialysis CKD who were anemic, various starting dose regimens of roxadustat were well tolerated and achieved anemia correction with reduced serum hepcidin levels. After anemia correction, hemoglobin was maintained by roxadustat at various dose frequencies without intravenous iron supplementation.
Keywords: C-Reactive Protein; Erythropoiesis; Hemoglobins; Hepcidins; Humans; Iron; Renal Insufficiency, Chronic; anemia; chronic kidney disease; clinical trial.
Copyright © 2016 by the American Society of Nephrology.
Figures

References
-
- Collins AJ, Ma JZ, Xia A, Ebben J: Trends in anemia treatment with erythropoietin usage and patient outcomes. Am J Kidney Dis 32[Suppl 4]: S133–S141, 1998 - PubMed
-
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 - PubMed
-
- Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW: Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med 316: 73–78, 1987 - PubMed
-
- Muirhead N: A rationale for an individualized haemoglobin target. Nephrol Dial Transplant 17[Suppl 6]: 2–7, 2002 - PubMed
-
- Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K: Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17: 1181–1191, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

