Hypoxia regulates global membrane protein endocytosis through caveolin-1 in cancer cells
- PMID: 27094744
- PMCID: PMC4842985
- DOI: 10.1038/ncomms11371
Hypoxia regulates global membrane protein endocytosis through caveolin-1 in cancer cells
Abstract
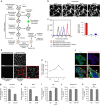
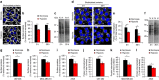
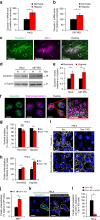
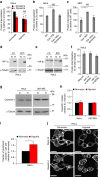
Hypoxia promotes tumour aggressiveness and resistance of cancers to oncological treatment. The identification of cancer cell internalizing antigens for drug targeting to the hypoxic tumour niche remains a challenge of high clinical relevance. Here we show that hypoxia down-regulates the surface proteome at the global level and, more specifically, membrane proteome internalization. We find that hypoxic down-regulation of constitutive endocytosis is HIF-independent, and involves caveolin-1-mediated inhibition of dynamin-dependent, membrane raft endocytosis. Caveolin-1 overexpression inhibits protein internalization, suggesting a general negative regulatory role of caveolin-1 in endocytosis. In contrast to this global inhibitory effect, we identify several proteins that can override caveolin-1 negative regulation, exhibiting increased internalization at hypoxia. We demonstrate antibody-mediated cytotoxin delivery and killing specifically of hypoxic cells through one of these proteins, carbonic anhydrase IX. Our data reveal that caveolin-1 modulates cell-surface proteome turnover at hypoxia with potential implications for specific targeting of the hypoxic tumour microenvironment.
Figures
Similar articles
-
Anti-chondroitin sulfate proteoglycan 4-specific antibodies modify the effects of vemurafenib on melanoma cells differentially in normoxia and hypoxia.Int J Oncol. 2015 Jul;47(1):81-90. doi: 10.3892/ijo.2015.3010. Epub 2015 May 18. Int J Oncol. 2015. PMID: 25997619 Free PMC article.
-
Distinct patterns of hypoxic expression of carbonic anhydrase IX (CA IX) in human malignant glioma cell lines.J Neurooncol. 2007 Jan;81(1):27-38. doi: 10.1007/s11060-006-9205-2. Epub 2006 Aug 31. J Neurooncol. 2007. PMID: 16944313
-
Three-dimensional culture promotes reconstitution of the tumor-specific hypoxic microenvironment under TGFβ stimulation.Int J Oncol. 2011 Nov;39(5):1327-36. doi: 10.3892/ijo.2011.1142. Epub 2011 Jul 22. Int J Oncol. 2011. PMID: 21785823
-
Carbonic anhydrase IX (CAIX) as a mediator of hypoxia-induced stress response in cancer cells.Subcell Biochem. 2014;75:255-69. doi: 10.1007/978-94-007-7359-2_13. Subcell Biochem. 2014. PMID: 24146383 Review.
-
Hypoxia-inducible factors in OSCC.Cancer Lett. 2011 Dec 26;313(1):1-8. doi: 10.1016/j.canlet.2011.08.017. Epub 2011 Aug 27. Cancer Lett. 2011. PMID: 21959110 Review.
Cited by
-
Organelle-Targeting Nanoparticles.Adv Sci (Weinh). 2025 Feb;12(7):e2411720. doi: 10.1002/advs.202411720. Epub 2025 Jan 13. Adv Sci (Weinh). 2025. PMID: 39806939 Free PMC article. Review.
-
Ion Channel Regulation in Caveolae and Its Pathological Implications.Cells. 2025 Apr 24;14(9):631. doi: 10.3390/cells14090631. Cells. 2025. PMID: 40358155 Free PMC article. Review.
-
Aza-BODIPY based carbonic anhydrase IX: Strategy to overcome hypoxia limitation in photodynamic therapy.Front Chem. 2022 Nov 2;10:1015883. doi: 10.3389/fchem.2022.1015883. eCollection 2022. Front Chem. 2022. PMID: 36405312 Free PMC article.
-
Phosphatidylinositol Monophosphates Regulate the Membrane Localization of HSPA1A, a Stress-Inducible 70-kDa Heat Shock Protein.Biomolecules. 2022 Jun 20;12(6):856. doi: 10.3390/biom12060856. Biomolecules. 2022. PMID: 35740982 Free PMC article.
-
Using quantitative single molecule localization microscopy to optimize multivalent HER2-targeting ligands.Front Med (Lausanne). 2023 Apr 17;10:1064242. doi: 10.3389/fmed.2023.1064242. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37138747 Free PMC article.
References
-
- Harris A. L. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–46 (2002). - PubMed
-
- Pouysségur J., Dayan F. & Mazure N. M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441, 437–443 (2006). - PubMed
-
- Mosesson Y., Mills G. B. & Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat. Rev. Cancer 8, 835–885 (2008). - PubMed
-
- Joffre C. et al. A direct role for Met endocytosis in tumorigenesis. Nat. Cell. Biol. 13, 827–837 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

